1216
A Shim Algorithm to Improve the Field Homogeneity and Image Quality in Cortico-Spinal fMRI1Department of Systems Neuroscience, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Synopsis
Cortico-spinal functional MRI covering a brain and a cervical spinal cord volume in the same acquisition, e.g. to investigate the interaction of brain and spinal cord areas, requires a dynamic shim update of the frequency and linear shim terms to obtain a reasonable EPI image quality in both volumes. Unfortunately, the optimum values for static higher-order and volume-specific dynamic linear shim terms cannot be determined with the standard shim algorithms provided by manufacturers. Here, a shim algorithm has been implemented that overcomes this problem and provides a better field homogeneity in the brain and spinal cord volumes.
Introduction
Cortico-spinal functional MRI covering a brain and a cervical spinal cord volume in the same acquisition, allows to investigate the interaction of brain and spinal cord areas, e.g. in pain processing or motor learning 1,2. Due to the large distance of the two target volumes, a reasonable image quality can only be achieved with a dynamic shim update between the volumes 1 which on most MR system is limited to the frequency and linear shim terms. This requires the joint optimization of volume-specific (dynamic) linear and static higher-order shim settings which cannot be performed by the standard shim algorithms of most manufacturers. Furthermore, finding a good approximation to this ideal solution involves a shim of a large volume covering both target regions to determine the higher-order shim terms and individual shims of the two target regions to determine their specific linear shim terms 1 yielding a rather time consuming procedure. In this study, a dedicated shim algorithm has been developed that determines optimized shim settings for global (static) second-order in combination with volume–specific (dynamic) linear shim terms. It shortens the shim procedure considerably and provides a better field homogeneity in the brain and spinal cord volumes compared to the conventional approach based on the manufacturer’s algorithm.Methods
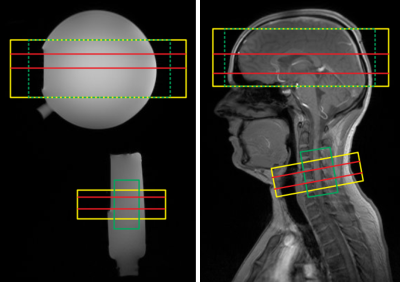
The shim algorithm for cortico-spinal fMRI (“CoSpi shim”) was implemented in IDL (L3Harris Geospatial, version 8.5.1). It requires a field map measurement (magnitude and phase difference of two echoes) and information on the two shim volumes as input. After a phase unwrap in each of the two shim volumes independently, it performs a fit of the obtained field map with second-order shim terms active in both volumes and two sets of constant and linear shim terms that are active only in the brain and spinal cord shim volume, respectively, but vanish in the other volume.Experiments were performed on a 3T whole-body MR system (Magnetom PrismaFit, Siemens, Germany) with a standard 64-channel head-neck coil on phantoms and healthy volunteers (Fig. 1) from which informed consent was obtained prior to the examination. Field maps were acquired using a 2D fast-gradient-echo technique with two echoes (echo times 4.26 ms and 6.72 ms) and a field-of-view of 256x256x400 mm3 covered with an isotropic resolution of 4.0 mm. FLASH images as anatomical reference and EPI images were acquired with resolutions of 2.0x2.0x2.0 mm3 (1.0 mm gap) in the brain volume (8 slices) and 1.0x1.0x5.0 mm3 in the spinal cord volume (24 slices), respectively.
The shim settings obtained with the conventional approach based on the manufacturers algorithm 1 and the CoSpi shim algorithm were compared with respect to the field inhomogeneity in the target volumes (standard deviation of the field map) and the EPI image quality.
Results and Discussion
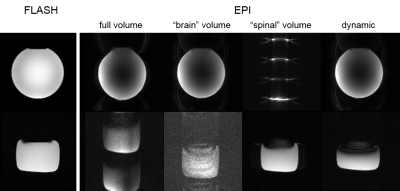
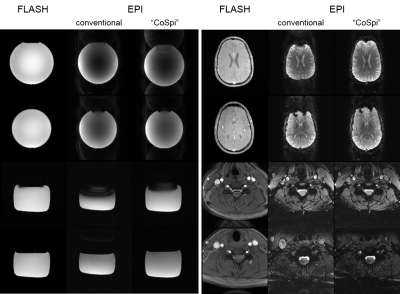
Figure 2 demonstrates the conventional shim approach and the need for a dynamic shim update for cortico-spinal fMRI. The higher-order shim terms are determined for the large adjustment volume covering both slice groups and the volume-specific linear terms from a shim adjusted in the corresponding target region (cf. Fig. 1). Only with a dynamic update of the linear shim terms between the two slice groups during the measurement, a reasonable image quality can be obtained in both volumes.In Fig. 3, field maps obtained in the phantom and in vivo with the conventional approach and the CoSpi algorithm are presented. The CoSpi algorithm provide a very similar or better field inhomogeneity with less phase wrappings in the phase difference images. This is also reflected in the standard deviations of the unwrapped field map that are reduced by about 30% / 80% (brain / spinal cord volume) in the phantom and 40% / 60% in vivo, respectively.
The EPI images shown in Fig. 4 are consistent with the findings of the field map measurements of Fig. 3. For instance, in the in vivo experiments, spinal cord slices appear very similar for both approaches but the brain slices are considerably compressed for the conventional shim approach which is in line with the anterior-posterior gradient of the phase differences (cf. Fig. 3). In the phantom experiments, compression and through-slice dephasing in the spinal cord volume is less pronounced for the CoSpi compared to the conventional approach that suffers from a field gradient in the slice-phase direction (cf. Fig. 3).
Overall, the CoSpi approach outperformes the conventional approach in terms of image quality. In addition, the time required for shimming is reduced from more than 15 min with the conventional approach to about 7 min with the CoSpi shim because only a single shim step is required.
In conclusion, the implemented algorithm could help to improve the performance and applicability of cortico-spinal fMRI.
Acknowledgements
No acknowledgement found.References
1. Finsterbusch J, Sprenger C, Büchel C. Combined T2*-weighted measurements of the human brain and cervical spinal cord with a dynamic shim update. NeuroImage. 2013; 79:153–161.
2. Sprenger C, Finsterbusch J, Büchel C. Spinal Cord–Midbrain Functional Connectivity Is Related to Perceived Pain Intensity: A Combined Spino-Cortical fMRI Study. J.Neurosci. 2015; 35(10):4248-4257.
Figures