1215
Motion-Compensated 3D TSE for More Robust Intracranial MR Vessel Wall Imaging1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Bioengineering Department, University of California, Los Angeles, Los Angeles, CA, United States, 3Siemens Healthineers, Los Angeles, CA, United States, 4A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 5Department of Radiology, Harvard Medical School, Boston, MA, United States, 6Department of Radiology, Fujian Medical University Union Hospital, Fuzhou, China, 7Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 8Department of Imaging, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 9Department of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
While underexplored to date, motion susceptibility may critically undermine clinical translation of 3D intracranial MR vessel wall imaging (VWI). Motion artifacts observed in intracranial VWI are either caused by head bulk motion or internally localized movement. By combing volumetric navigators (vNav) and self-gating (SG) strategies, we propose a novel motion compensation approach that can simultaneously address these two motion issues. Our preliminary studies demonstrated the potential of using this technique to improve robustness of 3D intracranial MR VWI.
Introduction
Intracranial MR vessel wall imaging (VWI) is the only non-invasive modality that can directly visualize the vessel wall and characterize wall pathologies, and has drawn great clinical interests in the past few years1. 3D variable-flip-angle turbo spin-echo (aka. SPACE, VISTA, or CUBE) is currently the method of choice for intracranial VWI. However, the 3D acquisition fashion and relatively long scan times render this method inherently susceptible to motion, which can result in the entire scan being corrupted to the extent that it is unusable2. Motion artifacts observed in intracranial VWI are either caused by head bulk motion (i.e. changing head position) or localized movement of internal anatomic structures (i.e. cough). The volumetric navigators (vNav) technique and self-gating (SG) strategy have demonstrated effectiveness in reducing motion artifacts caused respectively by these two motion types2,3. In this work, we develop a motion-robust intracranial MR VWI technique by incorporating a combined vNav-SG strategy into the SPACE sequence.Methods
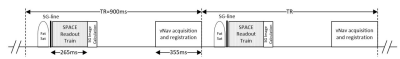
Sequence DesignA commercially available T1-weighted SPACE sequence was modified to include vNav and SG acquisition modules and corresponding real-time decision-making and communication modules (Figure 1). A center k-space line (SG line) is acquired from the first echo of the SPACE readout to derive the projection of the entire imaging volume. The projections acquired in subsequent TRs are cross-correlated (CC) to the reference projection collected at the beginning. Based on their CC values, which indicate the severity of motion contamination, all acquired TRs are prioritized and the most affected TRs are reacquired at the end of the scan. vNav was implemented as a 3D EPI module with 8-mm resolution and 256 mm FOV in all three directions3. We insert one such 3D navigator at the end of each TR to ensure that motion estimation is as close as possible to the SPACE readout train. Each subsequent navigator is registered back to the first navigator to realign the imaging coordinates of the SPACE readout and navigator.
In vivo Study
7 healthy volunteers and 3 ischemic stroke patients were recruited in the study. All scans were performed on a 3T system (MAGNETOM Skyra; Siemens Healthineers, Germany) with a 20-channel head-neck coil. For all healthy subjects, we performed directed-motion experiments to evaluate the improvement produced by our technique when imaging subjects who conduct both head bulk motion, i.e. changing head position, and localized movement, i.e. cough. All subjects underwent 6 scans using the developed vNav-SG SPACE sequence with different imaging conditions:
- Subject was asked to remain still; imaging without vNav or SG (denoted as “W/O MO, W/O vNav-SG”);
- Subject was asked to remain still; imaging with vNav and SG (“W/O MO, W/ vNav-SG”);
- Subject was asked to conduct motion; imaging without vNav or SG (“W/ MO, W/O vNav-SG”);
- Subject was asked to conduct motion; imaging with vNav only (“W/ MO, W/vNav”);
- Subject was asked to conduct motion; imaging with SG only (“W/ MO, W/SG”);
- Subject was asked to conduct motion; imaging with vNav and SG (“W/ MO, W/ vNav-SG”).
Data Analysis
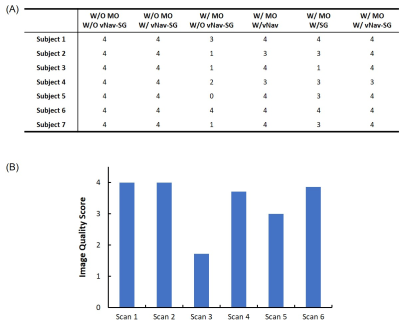
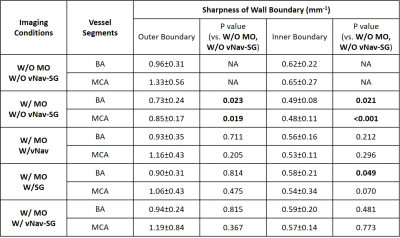
All the vNav-SG SPACE image sets were randomized and scored by a radiologist with 6 years of experience in neurovascular imaging to demonstrate the effect of motion on image quality and effectiveness of vNav-SG motion compensation technique in preventing quality deterioration. A five-point scale was used: 0-poor, 1-fair, 2-average, 3-good, 4-excellent. For quantitative analysis, vessel wall sharpness was measured at the inner and outer boundaries of 2 major vessel segments, i.e. middle cerebral arteries (MCA) and basilar arteries (BA). Reformatted 2D cross-sectional images were sharpness measured using an in-lab MATLAB sharpness measurement program2. The paired two-tailed Student’s t-test was used for the comparison between scans.
Results
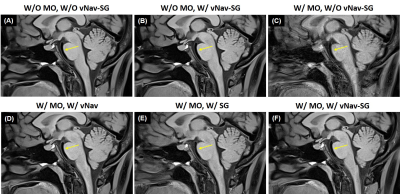
Figure 2 shows the images of the 6 imaging conditions in one healthy subject. Comparison of Figure 2A and 2B demonstrates that navigators and reacquisition did not affect image intensity or contrast. Motion artifacts led to severe degradation of overall image quality (Figure 2C) but were reduced by either vNav (Figure 2D) or SG (Figure 2E) and well suppressed by a combined vNav-SG strategy (Figure 2F). Motion significantly reduced overall image quality score and vessel wall sharpness at either outer or inner boundary, whereas the adoption of our vNav-SG technique significantly mitigated the effect (Figure 3 and Table 1). Figure 5 displays the post-contrast results in a representative stroke patient. Plaques (Figure 5B) and vessels (Figure 5D and 5F) can be clearly depicted with vNav-SG function on compared to their no motion-compensated counterparts (Figure 5A, 5C and 5E).Conclusion
Head bulk motion and localized movement can induce substantial artifactual effect in intracranial VWI, and a vNav-SG based motion compensation strategy is feasible in mitigating the motion artifacts and may help dramatically improve the robustness of the imaging modality in clinical settings.Acknowledgements
Granted by NIH/NHLBI 1R01 HL147355.References
1. Mandell D, Mossa-Basha M, Qiao Y, Hess C, Hui F, Matouk C, Johnson M, Daemen M, Vossough A, Edjlali M. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. American Journal of Neuroradiology 2017;38(2):218-229.
2. Fan Z, Zuehlsdorff S, Liu X, Li D. Prospective self‐gating for swallowing motion: A feasibility study in carotid artery wall MRI using three‐dimensional variable‐flip‐angle turbo spin‐echo. Magnetic resonance in medicine 2012;67(2):490-498.
3. Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJ. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magnetic resonance in medicine 2012;68(2):389-399.
Figures