1213
Reproducibility, Repeatability and Preliminary Clinical Results of Dixon Cardiac MRF: T1, T2, ECV and fat fraction tissue characterization1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Philips Healthcare, London, United Kingdom, 3Philips Research Hamburg, Hamburg, Germany
Synopsis
Dixon cardiac Magnetic Resonance Fingerprinting (DcMRF) has been recently proposed to enable simultaneous water T1, water T2 and fat fraction (FF) quantification in a single breath-hold scan. Here we investigate the reproducibility, repeatability and clinical feasibility of DcMRF in comparison to reference MOLLI, T2GRASE and 6 echo proton density FF measurements. Reproducibility and repeatability were investigated in healthy subjects, whereas native T1, T2 and FF, and post contrast T1 and synthetic ECV measurements were performed in patients with suspected cardiovascular disease.
Introduction
Dixon cardiac Magnetic Resonance Fingerprinting (DcMRF1) has been recently proposed for fast and simultaneous multi-parametric mapping of water T1, water T2 and fat fraction (FF). T1, T2 and FF are promising biomarkers for the assessment of a large range of cardiac pathologies including detection of fibrosis, edema, inflammation and fat infiltration. Here we investigate the reproducibility, repeatability and preliminary clinical feasibility of DcMRF in comparison to conventional mapping techniques.Methods
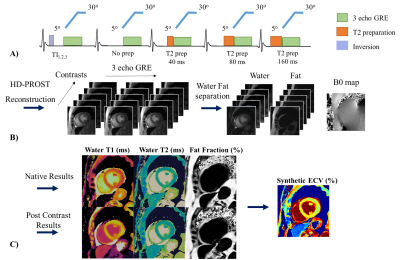
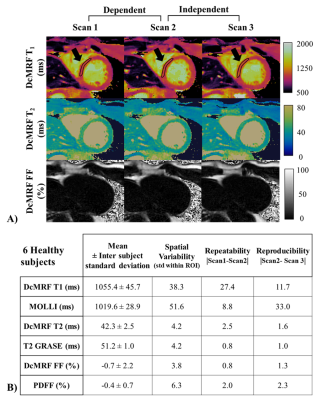
The DcMRF framework is shown in Figure 1. Acquisition parameters include 15 modules with varying magnetization preparation (Fig.1A), 29 TRs per module and three bipolar readouts per TR, TR/TE1/ΔTE= 7.5/1.6/1.93ms, 1.8x1.8mm2 resolution, 8mm slice thickness, FOV= 547x547 mm2. Images were reconstructed using HD-PROST2, separated into water and fat images1,3 and matched to a dictionary using the inner-product4 (Fig.1B). DcMRF, T1 (MOLLI), T2 (T2GRASE) and proton density FF (PDFF, 6 echoes GRE) measurements were performed on a 1.5T scanner (Ingenia, Philips Healthcare) in 6 healthy subjects (2 females, age: 29 ± 4.7 yo, heartrate: 70 ± 11.2 bpm) in short-axis. Each subject was scanned three times in the same session. Scans 1 and 2 (dependent scans) were performed sequentially, whereas for scan 3 (independent scan)5,6 the subject was removed from the scanner and repositioned on the table. Repeatability and reproducibility are obtained comparing parameter measurements’ absolute differences between scans 1 and 2, and between scans 2 and 3, respectively. DcMRF, MOLLI, T2GRASE and PDFF short axis images were acquired in 10 patients (4 females, age: 61.1 ± 12.4 yo, heartrate: 70.8 ± 9.1) with suspected cardiovascular disease. DcMRF and MOLLI were performed both before and after gadolinium-based contrast injection. The same acquisition was performed for DcMRF pre- and post-contrast. A 5(3)3 scheme was used for pre-contrast MOLLI, whereas a 4(1)3(1)2 scheme was employed after contrast injection, as per clinical guidelines in our institution. PSIR late gadolinium enhancement (LGE) images were also obtained in patients, as per clinical protocol, for scar visualization. Additional views (e.g. 4-chamber, 2-chamber or apical views) were acquired using DcMRF post-contrast in the case of pathology findings on PSIR LGE images. A synthetic haematocrit Hctsyn was computed from the MOLLI native blood T1 value7 and used for (synthetic) extra-cellular volume (ECV) quantification (of both MOLLI and DcMRF) (Fig.1C). For analysis, regions of interest (ROIs) were manually drawn in the septum or in the diseased and remote areas when relevant for patients.Results
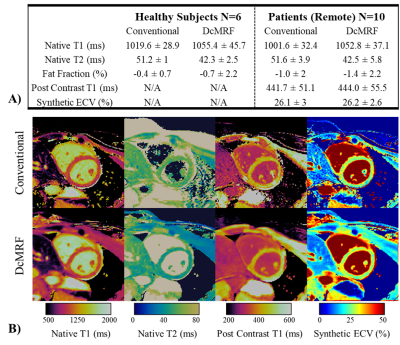
T1, T2 and FF DcMRF maps are shown in Fig.2A for a representative healthy subject for the 3-scan session. Mean, inter-subject standard deviation, spatial variability (within region standard deviation), repeatability and reproducibility for T1, T2 and FF measurements with DcMRF in comparison to conventional approaches are reported in Fig.2B. These preliminary results show good precision of the technique (mean repeatability and reproducibility <30ms for T1, <3ms for T2 and <2% for FF) with slight biases compared to MOLLI and T2GRASE, consistent with previous cardiac MRF findings8,9. Slightly negative FF values were observed with DcMRF (and PDFF) when estimating very low fat content. These errors may be due to residual motion, the lack of T2* correction10 and phase errors11.Healthy subjects’ T1, T2 and FF septum measurements, patient remote mean measurements and inter-subject standard deviation are shown in Fig.3A for DcMRF and the corresponding conventional techniques. These preliminary results show similar biases for native measurements between techniques for the healthy subjects’ and patients’ cohorts. Similar mean post contrast T1 (+2.3ms compared to MOLLI) and synthetic ECV (+0.1% compared to MOLLI based synthetic ECV) values were obtained in patients between techniques.
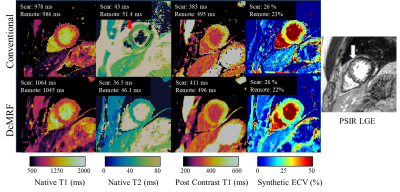
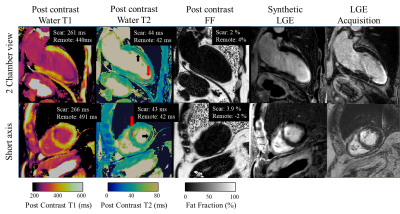
Native T1, native T2, post-contrast T1 and ECV are shown in Fig.3B for both DcMRF and conventional techniques in a patient with no particular finding. Native T1, native T2, post-contrast T1 and ECV are shown in Fig.4 for both DcMRF and conventional techniques in a patient with scar (infarction occurred ~1 year prior and observed in the PSIR LGE image). Corresponding values measured in the scar and remote areas are included at the top of each map. Post-contrast DcMRF T1, T2 and FF maps are shown in Fig.5 for an additional patient. The patient presented with myocardial infarction and non-viable myocardium in the left anterior descending artery territory. A synthetic fat-suppressed PSIR LGE image can be generated from the proposed DcMRF as shown in Fig.5 in comparison to the clinical PSIR LGE image. No fat infiltration, no T2 increase, but a decrease in post-contrast T1 in the scar region compared to remote tissue (consistent with a chronic infarct12) is observed with DcMRF.
Conclusion
DcMRF provides reproducible T1, T2 and FF measurements in healthy subjects in a single breath-hold. Preliminary clinical evaluation shows that DcMRF achieves comparable results to conventional sequentially acquired techniques, but in a single breath hold and providing intrinsically co-registered maps. Future work will focus on further assessing DcMRF in patients with diverse pathophysiologies such as fat infiltrations, inflammation, tumors and/or scar. The reproducibility and repeatability study will also be strengthened through additional recruitment of healthy subjects and statistical analysis.Acknowledgements
This work was supported by EPSRC (EP/L015226/1, EP/P001009/1, EP/P032311/1) and Wellcome EPSRC Centre for Medical Engineering (NS/ A000049/1).References
1. Jaubert, O. et al. Water/Fat Dixon Cardiac Magnetic Resonance Fingerprinting. Magnetic Resonance in Medicine In Press, (2019).
2. Bustin, A. et al. High-Dimensionality Undersampled Patch-Based Reconstruction (HD-PROST) for Accelerated Multi-Contrast Magnetic Resonance Imaging. Magnetic Resonance in Medicine 81, 3705–3719 (2019).
3. Berglund, J., Johansson, L., Ahlström, H. & Kullberg, J. Three-point dixon method enables whole-body water and fat imaging of obese subjects. Magnetic Resonance in Medicine 63, 1659–1668 (2010).
4. Ma, D. et al. Magnetic resonance fingerprinting. Nature 495, 187–92 (2013).
5. Roujol, S. et al. Accuracy, Precision, and Reproducibility of Four T1 Mapping Sequences: A Head-to-Head Comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology 272, 683–689 (2014).
6. Kvernby, S., Warntjes, M., Engvall, J., Carlhäll, C.-J. & Ebbers, T. Clinical feasibility of 3D-QALAS – Single breath-hold 3D myocardial T1- and T2-mapping. Magnetic Resonance Imaging 38, 13–20 (2017).
7. Fent, G. J. et al. Synthetic Myocardial Extracellular Volume Fraction. JACC: Cardiovascular Imaging 10, 1402–1404 (2017).
8. Hamilton, J. I. et al. MR fingerprinting for rapid quantification of myocardial T1, T2, and proton spin density. Magnetic Resonance in Medicine 77, 1446–1458 (2017).
9. Hamilton, J. I. et al. Investigating and reducing the effects of confounding factors for robust T1 and T2 mapping with cardiac MR fingerprinting. Magnetic Resonance Imaging 53, 40–51 (2018).
10. Reeder, S. B. & Sirlin, C. B. Quantification of liver fat with magnetic resonance imaging. Magnetic resonance imaging clinics of North America 18 (3), 337–57 (2010).
11. Hernando, D., Hines, C. D. G., Yu, H. & Reeder, S. B. Addressing phase errors in fat-water imaging using a mixed magnitude/complex fitting method. Magnetic resonance in medicine 67, 638–44 (2012).
12. Tahir, E. et al. Acute versus Chronic Myocardial Infarction: Diagnostic Accuracy of Quantitative Native T1 and T2 Mapping versus Assessment of Edema on Standard T2-weighted Cardiovascular MR Images for Differentiation. Radiology 285, 83–91 (2017).
Figures