1212
Retrospective Analysis of Pilot Tone Derived Cardiac and Respiratory Motion Information in a Patient Cohort1Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 2Siemens Healthineers, Erlangen, Germany, 3Siemens Healthineers, Lausanne, Switzerland
Synopsis
The Pilot Tone is a novel motion sensing method capable of simultaneous, sequence independent measurement of respiratory and cardiac motion. Here, we show that Pilot Tone motion data can be used as an alternative to self-gating in a free-breathing, cardiac- and respiration resolved CMRI sequence. We demonstrate in a patient cohort that Pilot Tone motion information correlates well with ECG ground-truth and self-gating respiratory signals.
Introduction
The Pilot Tone navigator1 is a novel motion sensing method capable of measuring physiological motion. A weak high-frequency magnetic field $$$\mathbf{B}_{PT}$$$ is generated within the bore, which is then modulated by the changing electromagnetic environment due to changes in tissue distribution from respiration and cardiac activity. The frequency $$$\omega_{PT}$$$ is chosen outside the imaging bandwidth yet within the operating bandwidth of the MR receiver. Thus, the modulated field $$$\mathbf{B}_{PT}$$$ can be measured in each channel using the existing receiver hardware to obtain the multidimensional Pilot Tone signal $$$\mathbf{S}_{PT}(t)$$$. We have previously demonstrated that individual channels can be linearly combined in such a way as to separate cardiac and respiratory motion2. We now show that this motion information can be used instead of self-gating in a free-breathing, cardiac- and respiration resolved 5D MRI reconstruction.Methods
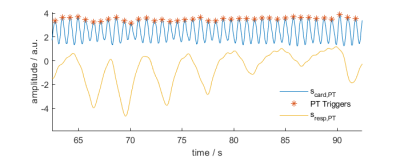
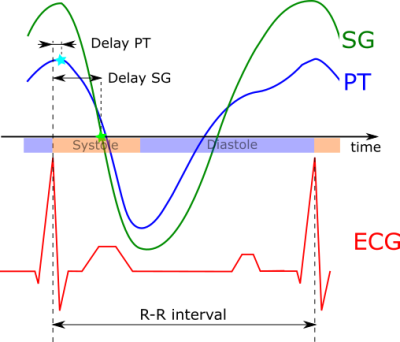
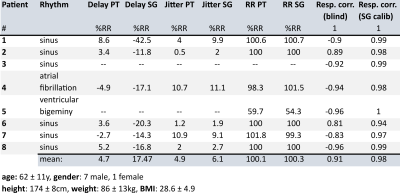
Eight patients were scanned at 1.5T in an MRI scanner with integrated PT generator (MAGNETOM Sola with Biomatrix Body12 coil, Siemens Healthcare, Erlangen, Germany) using a prototype free-running sequence3 based on a 3D radial acquisition4 (TE: $$$1.56\,ms$$$, TR: $$$72.43\,ms$$$, FoV: $$$220\,mm$$$, $$$2\,mm^3$$$ resolution, scan-time: $$$2.4\,min$$$). The cardiac- and respiratory motion signals $$$s_{card,PT}(t)$$$ and $$$s_{resp,PT}(t)$$$ were extracted from $$$\mathbf{S}_{PT}(t)$$$ blindly, i.e. without any additional information using Independent Component Analysis (FastICA5) and Principal Component Analysis (PCA) respectively. To eliminate any residual respiratory motion component in $$$s_{card,PT}(t)$$$ and for de-noising, an IIR forward/backward bandpass-filter ($$$f_{c,lo}=0.7\,Hz$$$, $$$f_{c,hi}=6\,Hz$$$) was applied to $$$s_{card,PT}(t)$$$. Trigger timepoints were then found using Matlab’s findpeaks function as local maxima of $$$s_{card,PT,filt}(t)$$$ (see Fig. 1). The extraction and processing of the SG signal is detailed as part of the fully self-gated (SG) free-running framework in Di Sopra et al6. Note that the triggering-algorithm in [6] uses zero-crossings of the SG cardiac signal instead of local maxima.Triggers from PT and SG were compared to ECG ground-truth in terms of trigger-delay (temporal distance from ECG triggers), trigger-jitter (one standard-deviation of trigger-delay) and R-R interval (see Fig. 2). To facilitate comparability between patients, all time-measurements are given as normalized values in percent of the patients mean R-R interval from ECG.
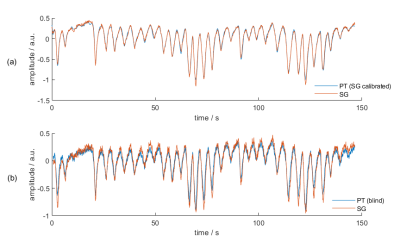
Correlation between PT respiratory signals obtained from PCA (blind) and SG respiratory signals $$$s_{resp,SG}(t)$$$ (Fig. 3b) are reported in Tab. 1. Additionally, we attempted to find for PT data the linear combination that best matches $$$s_{resp,SG}(t)$$$, i.e. the superior-inferior (SI) component of respiration, by solving for
$$\min_x \left| \mathbf{x} \mathbf{S}_{PT}-s_{resp,SG} \right|$$ (see Fig. 3a)
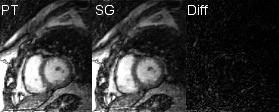
To demonstrate equivalency between cardiac and respiratory binning information from PT and SG, two datasets were reconstructed using a prototype reconstruction algorithm6 from PT and SG motion information respectively (Fig. 4).
Results
From 8 scanned patients, two had to be excluded from analysis as ECG recording failed (see Tab. 1). On average, trigger points from local maxima of $$$s_{card,PT}$$$ correspond very well to ECG R-peak (mean absolute delay with respect to R-peak: PT:$$$\,4.73\,\%RR$$$, SG:$$$\,17.47\,\%RR$$$) and show slightly less jitter than SG derived triggers (PT:$$$\,4.89\,\%RR$$$, SG:$$$\,6.12\,\%RR$$$). Mean R-R intervals from both PT and SG correspond very well to ECG ground-truth (PT:$$$\,100.12\,\%RR$$$, SG:$$$\,100.25\,\%RR$$$). PT respiratory signals found by PCA correlated well to those from SG (mean absolute correlation:$$$\,0.91$$$) and PT data were found to contain the specific SG respiratory signal almost exactly (mean correlation:$$$\,0.98$$$). Images reconstructed from $$$\mathbf{S}_{PT}$$$ (cardiac and respiration from blind separation) appear visually similar and comparable in sharpness to those reconstructed from SG data (see Fig. 4).Discussion
We demonstrated that motion information obtained from PT correlates well to that from self-gating both for cardiac and respiratory motion and can be used to retrospectively bin and reconstruct 5D motion-resolved free-running acquisitions while offering the advantage of sequence independent high sampling rate, avoiding the need for navigator readouts or periodic SI-projections. This potentially offers enhanced flexibility in the design of k-space trajectories.We hypothesize that the PT signal is linked to cardiac volume, prompting the use of local maxima as trigger points which, under this assumption, should mark the onset of mechanical contraction. Results seem to confirm this hypothesis, with local maxima appearing close to ECG R-peak and, on average, reduced trigger-jitter compared to SG.
$$$\mathbf{S}_{PT}$$$ was previously found to contain multi-dimensional respiratory motion information: By calibrating to some seconds of S-I and A-P image navigators, motion curves corresponding to movement of both chest wall and liver dome can be extracted from $$$\mathbf{S}_{PT}$$$7. In our cohort, the strongest principal component of $$$\mathbf{S}_{PT}$$$ yields strong correlation to the respiratory signal from SG, but does not perfectly match it. However, the information of $$$s_{resp,SG}(t)$$$ can be almost perfectly reconstructed from $$$\mathbf{S}_{PT}$$$ showing that the information is, in principle, contained in $$$\mathbf{S}_{PT}$$$. This multidimensional information could be utilized in the future by e.g. obtaining the S-I and A-P respiratory signals in a short calibration scan that precedes the image acquisition.
Conclusion
The Pilot tone navigator is a very attractive alternative to SG for fully self-gated free-running MRI as it allows for accurate respiratory and cardiac binning prior to compressed sensing reconstruction. This concept further removes the need for ECG lead placement and R-wave triggering, and it can be used completely independent of sequence parameters and acquisition schemes, allowing for more flexibility in the design of sampling patterns.Acknowledgements
References
[1] P. Speier et al. PT-Nav: A Novel Respiratory Navigation Method for Continuous Acquisition Based on Modulation of a Pilot Tone in the MR-Receiver, Proc. ESMRMB 129:97-98, 2015.
[2] M. Bacher et al. Retrospective Evaluation of Pilot Tone Based Cardiac Trigger Quality In A Volunteer Cohort, Book of Abstracts ESMRMB 2017 30:360- 361.
[3] S. Coppo et al., Free‐running 4D whole‐heart self‐navigated golden angle MRI: Initial results, Magn. Reson. Med., 74: 1306-1316. doi:10.1002/mrm.25523
[4] D. Piccini et al. Spiral Phyllotaxis: The Natural Way to Construct a 3D Radial Trajectory in MRI. Magnetic Resonance in Medicine 2011, 66, 1049–1056.
[5] A. Hyvärinen et al.: A Fast Fixed-Point Algorithm for Independent Component Analysis. Neural Computation, 9(7):1483-1492, 1997
[6] L. Di Sopra et al., An Automated Approach to Fully Self-Gated Free-Running Cardiac and Respiratory Motion-Resolved 5D Whole-Heart MR Imaging. Magn Reson Med 2019, In Press
[7] L. Schröder et al. “Two-Dimensional Respiratory-Motion Characterization for Continuous MR Measurements Using Pilot Tone Navigation”, Proceedings of the 24th Annual Meeting of the ISMRM ﴾ISMRM 2016﴿, #1876
Figures