1211
The relationship between CMR–derived myocardial strain and late gadolinium enhancement in asymptomatic heart transplant patients1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China, 2MR Collaboration, Siemens Healthineers Ltd., Shanghai, China, Shanghai, China, 3Department of Ultrasound, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Synopsis
No study has explored the relationship between CMR-derived myocardial strain and the extent of LGE in asymptomatic HT patients. The purpose of this study was to evaluate this relationship using DRA and FT strain analysis. In this study, there were strong correlation and good reproducibility between DRA and FT strain modalities. HT patients with LGE had reduced LVGLS and preserved LVGCS. CMR-derived LVGLS was significantly and independently correlated with LGE.
Introduction
Late gadolinium enhancement (LGE) imaging with cardiovascular magnetic resonance (CMR) can accurately and precisely detect regional myocardial infarction and fibrosis. Regional myocardial infarction and fibrosis have been shown to be crucial independent predictive factors of poor prognosis, including major adverse cardiac events and mortality in heart transplant (HT) patients1,2, as well as in patients with other heart diseases3,4. CMR-derived myocardial strain parameter assessment has been proposed as a non-invasive method for quantifying myocardial deformation and is recommended in adult guidelines for HT patients to detect subclinical allograft dysfunction5. However, no study investigates the relationship between CMR-derived strain parameters and LGE in asymptomatic HT patients. In this study, we (1) explored the correlation between deformation registration algorithm (DRA) and feature tracking (FT) strain analysis; (2) determined the inter- and intra-observer agreement between both strain modalities; (3) described the relationship between CMR-derived myocardial strain parameters and LGE in asymptomatic HT patients.Methods
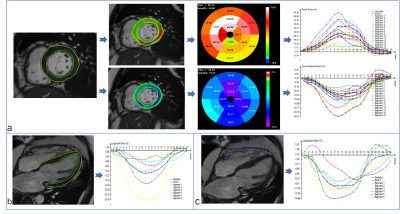
Between September 2018 and July 2019, 72 HT patients underwent CMR scans. The exclusion criteria included reduced left ventricular ejection fraction (LVEF) (<50%), chronic atrial fibrillation, current histologically or clinically confirmed significant acute rejection, severe renal insufficiency, and contraindications to the CMR exam. All subjects underwent standard CMR examinations with a 1.5T MR scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). A balanced steady-state free-precession sequence was performed to acquire LV long-axis cine (2-, 3-, 4-chamber) and a stack of short-axis cines. The prototype software (TrufiStrain, version 2.0, Siemens Healthcare, Erlangen, Germany) and the commercial software (cvi42, Circle, Calgary, Canada) were used for DRA and FT strain analysis, respectively. For DRA analysis, LV global longitudinal strain (LVGLS) was measured from the standard 4-chamber image; LV global radial strain (LVGRS), and LV global circumferential strain (LVGCS) were measured from 3 short-axis images (base, middle and apex). The right ventricular longitudinal strain (RVLS) was obtained from the RV free wall in a standard 4-chamber image (Figure 1). For FT analysis, LVGLS was derived from long-axis slices (including 2-, 3- and 4- chamber). LVGCS and LVGRS were all taken from short slices, and the acquisition of RVLS was acquired as in the DRA analysis (Figure 2). A threshold 4 standard deviations (SD) above the mean signal intensity of the remote normal myocardium from the same slice was used to quantify LGE6.Results
There was a significant correlation between DRA and FT strain modalities (r=0. 674 for LVGLS, r=0.73 for LVGRS, r=0.811 for LVGCS, and r=0.724 for RVLS, all P < 0.001). The intraclass correlation coefficient and Bland-Altman analyses were used to measure observer agreements and limits of agreement (LOA) of strain parameters. Intra- and interobserver agreements of all myocardial strain for DRA and FT analyses were excellent (greater than 0.9). In addition, DRA exhibited better observer agreement and narrower LOA in all strain parameters.Using these two techniques, we showed that 25 HT patients with LGE had lower LVGLS and similar LVGCS than the patients without LGE (Figure 3). In the receiver operating characteristic (ROC) analysis, the under the curve (AUC) for the DRA-LVGLS and FT-LVGLS were 0.666 and 0.733, respectively. The logistic regression showed LVGLS from both techniques was significantly associated with LGE (odds ratios [OR] =1.376 for DRA-LVGLS; OR=1.402 for FT-LVGLS).Discussion
DRA and FT strain modalities had significant correlation and high inter- and intra-reproducibility. Compared to FT, DRA had better observer agreement and narrower LOA In our study, the HT patients with LGE had lower LVGLS than the patients without LGE, and this strain parameter was significantly and independently associated with the extent of LGE, whereas the LVGCS measurements obtained with both methods was not. These results contrast with findings from a previous study of a much smaller sample size in which only 5/20 HT patients with LGE showed no correlation between LGE and LVGLS8. Moreover, several studies9,10 have reported that reduced LVGLS and LVGCS values were associated with LGE in other heart diseases; interestingly, the relationship between LVGCS and LGE was stronger than that between LVGLS and LGE. This is completely different from our results, which may be related to the development of these diseases and the distribution of LGE.Conclusions
DRA and FT strain analysis in HT patients demonstrated strong correlation and excellent observer agreement. CMR-derived LVGLS was the strain parameter significantly and independently correlated with LGE in HT patients.Acknowledgements
Not applicable.References
1. Pedrotti P, Vittori C, Facchetti R, et al. Prognostic impact of late gadolinium enhancement in the risk stratification of heart transplant patients. Eur Heart J Cardiovasc Imaging 2017;18(2):130–137.
2. Hughes A, Okasha O, Farzaneh-Far A, et al. Myocardial Fibrosis and Prognosis in Heart Transplant Recipients. Circ Cardiovasc Imaging. 2019;12(10):e009060.
3. Shanbhag SM, Greve AM, Aspelund T, et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur Heart J. 2019;40(6):529-538.
4. Gräni Christoph, Eichhorn C, Bière Loïc, et al. Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis[J]. Journal of Cardiovascular Magnetic Resonance, 2019, 21(1).
5. Badano LP, Miglioranza MH, Edvardsen T, et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur Heart J Cardiovasc Imaging 2015;16:919–948.
6. Beliveau P, Cheriet F, Anderson SA, Taylor JL, Arai AE, Hsu LY. Quantitative assessment of myocardial fibrosis in an age-related rat model by ex vivo late gadolinium enhancement magnetic resonance imaging with histopathological correlation. Comput Biol Med. 2015;65:103–13.
7. Narang A , Blair J E , Patel M B , et al. Myocardial perfusion reserve and global longitudinal strain as potential markers of coronary allograft vasculopathy in late-stage orthotopic heart transplantation[J]. The International Journal of Cardiovascular Imaging, 2018, 34(10),1607-1617.
8. Altiok E , Tiemann S , Becker M , et al. Myocardial Deformation Imaging by Two-Dimensional Speckle-Tracking Echocardiography for Prediction of Global and Segmental Functional Changes after Acute Myocardial Infarction: A Comparison with Late Gadolinium Enhancement Cardiac Magnetic Resonance[J]. Journal of the American Society of Echocardiography, 2014, 27(3):249-257.
9. Erley J , Genovese D , Tapaskar N , et al. Echocardiography and cardiovascular magnetic resonancebased evaluation of myocardial strain and relationship with late gadolinium enhancement[J]. Journal of Cardiovascular Magnetic Resonance, 2019, 21(1):46.
Figures