1206
CrCEST energetics of calf muscle groups at 3T distinguish patients with peripheral arterial disease from age-matched normals1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Cardiology, University of Virginia, Charlottesville, VA, United States, 3Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States
Synopsis
In Peripheral Arterial Disease, arterial occlusions in the lower limbs lead to tissue ischemia, which can lead to claudication pain or the need for amputation. Creatine CEST (CrCEST) imaging can indirectly image creatine as it exchanges protons with free water during metabolism after exercise. Ischemia in the skeletal reduces available oxygen, slowing metabolism. CrCEST data of three major calf muscle groups were fit to a monoexponential function in order to compare energetics between PAD patients and age-matched normal subjects. We have so far imaged 22 aged-matched subjects and 19 PAD patients, and found a significant increase in the decay constant.
Introduction
Peripheral arterial disease (PAD) is an atherosclerotic disease affecting over 200 million people worldwide. In PAD, arterial occlusions in the lower limbs lead to tissue ischemia, which can lead to claudication pain and the need for revascularization or amputation. 31Phosphorous (31P) spectroscopy has been used to assess phosphocreatine metabolism in the skeletal muscle of the calf, but it requires specialized coils, and suffers from low SNR and non-spatial data. Creatine chemical exchange saturation transfer (CrCEST) imaging uses standard 1H coils to indirectly image creatine as it exchanges protons with free water during phosphocreatine metabolism after exercise. Ischemia in the skeletal muscle of the calf reduces available oxygen, slowing the rephosphorylation of creatine in the mitochondria. We hypothesize that CrCEST creatine decay constants can distinguish these groups. Spatial information on metabolic function can aid in disease staging and treatment planning by showing tissue function beyond perfusion metrics like the ankle brachial index (ABI).Methods
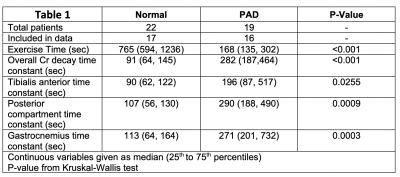
We have applied CrCEST in 22 healthy subjects and 19 PAD patients who performed plantarflexion ergometry to exhaustion or claudication. Creatine levels were measured over 10 min using a pulse sequence from the Center for MR and Optical Imaging at the University of Pennsylvania on a Siemens 3T Prisma [2,3]. Water saturation with shift reference (WASSR) and B1 maps were collected for B0 and B1 correction. Six images were acquired over 24 s intervals with saturation frequency offsets of ±1.3, ±1.8, and ±2.3 ppm. A 500 ms saturation pulse train was applied consisting of five 99.6 ms Hanning windowed pulses with 150 Hz B1 amplitude separated by a 0.4 ms inter-pulse delay. A fat saturation pulse was applied, followed by a single-shot spoiled gradient-echo readout with centric encoding, flip angle 10°, FOV 160x160 mm, matrix 128x128, TR 6.0 ms, TE 3 ms, slice thickness 10mm. ROIs containing the tibialis anterior, posterior compartment, and gastrocnemius of the calf were drawn by hand for each subject. Values of the mean CrCEST asymmetry signal of each ROI at each acquisition time were fit to a monoexponential with a decay constant τ.Results
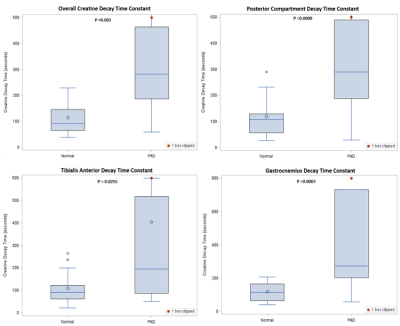
Of the age-matched normal subjects, four were excluded due to failure to reach exhaustion from exercise, and one was excluded due to coil failure during scanning. All PAD patients had analyzable data, however three patients had an initial rise in creatine concentration from baseline after exercise. This caused the energetics to be better fit to the negative of a monoexponential decay, and were thus excluded. This could possibly be caused by a perfusion delay or altered mitochondrial function due to chronic ischemia [4,5,6]. ROIs of containing the tibialis anterior, posterior compartment, gastrocnemius, and all muscles (excluding the bones) were compared in the remaining 17 normal subjects and 16 PAD patients. This data is shown in Table 1 and Figure 1, and P-values were calculated using Kruskal-Wallis test. The overall average decay time was 91 seconds in the age-matched normal subjects, and 282 in the PAD patients.Conclusion
CrCEST imaging is an emerging method capable of providing spatial image data on metabolism. Analysis on the muscle group level in patients with PAD significantly distinguishes them from age-matched normal subjects. Variation in decay time and shape was much higher in PAD patients than normal subjects, suggesting heterogeneity of disease severity and function among patients. Metabolic information could be used in conjunction with perfusion metrics like the ABI or MR perfusion measures for improved diagnostics and treatment planning. The next steps of our study involve same day comparison of CrCEST with 31P MRS, and CrCEST imaging before and after surgical and endovascular revascularization procedures.Acknowledgements
No acknowledgement found.References
[1] Ostchega, Y, et al.Journal of the American Geriatrics Society 2007; 55: 583-589.
[2] Haris M, Singh A, Cai K, et al. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat Med. 2014;20(2):209–214. doi:10.1038/nm.3436
[3] Kogan F, Haris M, Debrosse C, et al. In vivo chemical exchange saturation transfer imaging of creatine (CrCEST) in skeletal muscle at 3T. J Magn Reson Imaging. 2014;40(3):596–602. doi:10.1002/jmri.24412
[4] Kramer CM. Skeletal muscle perfusion in peripheral arterial disease a novel end point for cardiovascular imaging. JACC Cardiovasc Imaging. 2008;1(3):351–353. doi:10.1016/j.jcmg.2008.03.004
[5] Nygren, Anders & MD, PhD. (2006). Delayed contrast agent kinetics in ischemic skeletal muscle. J Magn Reson Imaging. 23. 171 - 176. 10.1002/jmri.20482.
[6] Andreas, M., Schmid, A.I., Keilani, M. et al. Effect of ischemic preconditioning in skeletal muscle measured by functional magnetic resonance imaging and spectroscopy: a randomized crossover trial. J Cardiovasc Magn Reson 13, 32 (2011) doi:10.1186/1532-429X-13-32
Figures