1202
Microstructural cardiac remodelling in aortic stenosis and its reversibility following valve replacement – a CMR diffusion tensor imaging study1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland, 2Department of Cardiology, University Hospital Zurich, Zurich, Switzerland, 3Great Ormond Street Hospital, London, United Kingdom, 4Institute of Diagnostic and Interventional Radiology, University Hospital Zurich, Zurich, Switzerland, 5Division of Cardiology, Pulmonology and Vascular Medicine, University Hospital Duesseldorf, Duesseldorf, Germany, 6Department of Cardiac Surgery, University Hospital Zurich, Zurich, Switzerland
Synopsis
CMR diffusion tensor imaging (CMR DTI) allows for the assessment of cardiac microstructure in diseased hearts. We investigated changes of myocardial diffusion properties and myocyte orientation in patients with aortic stenosis (AS) before and after valve replacement (AVR) using DTI and T1-mapping. Mean diffusivity (MD), fractional anisotropy (FA), E2A sheet angle and the transmural helix angle (HA)-slope were altered in AS patients, while native T1 was not significantly different. After AVR, the HA-slope was the only parameter with reversible changes, whereas MD, FA and E2A remained abnormal. This study indicates that AS-induced alterations of myocardial microstructure partly persist following AVR.
Introduction
Aortic stenosis (AS) is the most prevalent valvular heart disease and associated with a high mortality when symptoms occur1. However, even after timely aortic valve replacement (AVR), patients have shorter life expectancy compared to the general population2. This may be due to adverse cardiac remodelling such as diffuse and focal fibrosis which are already present at the time of intervention and do not resolve after AVR3. Therefore, a detailed understanding of the reversibility of AS-induced myocardial remodelling may help to optimize treatment planning. CMR diffusion tensor imaging (CMR DTI) is a versatile tool to assess the cardiac microstructure and myocardial fibre orientation4,5. The objective of the present work was to investigate changes in myocardial microstructure due to AS and their reversibility after AVR using CMR DTI.Methods
Ten AS patients without other cardiac disease and 10 controls were prospectively enrolled in this ongoing study. Patients who underwent AVR were scheduled for a follow-up CMR approximately 6 months after surgery. All examinations were performed on a 1.5T Philips Achieva system (Philips Healthcare, Best, The Netherlands) equipped with a 5 channel receiver coil. The study protocol was approved by the local ethics committee and written informed consent according to institutional guidelines was obtained prior to imaging.For CMR DTI, a diffusion-weighted second-order motion compensated spin-echo (SE) sequence was acquired6. Diffusion imaging was ECG-triggered to mid systole and performed under free breathing with navigator-based slice tracking7. Diffusion weighting was encoded along 3 and 9 directions with b=100/450 s/mm2, respectively (resolution: 2.5×2.5×8 mm3, FOV: 230×98 mm2, TE/TR: 76 ms/3-R-R)8. DTI analysis was performed on mean diffusivity (MD), fractional anisotropy (FA), helix and absolute E2A sheet angle (HA, E2A) as well as the transmural HA slope.
Cardiac volumes, mass and systolic function were assessed by a contiguous stack of short-axis cine images covering the left ventricle. T1 mapping was performed at the same slice location as the diffusion scans using a modified look-locker inversion recovery (MOLLI) technique9 with a 5(3)3 scheme. Per slice, 8 T1-weighted images over 11 heartbeats were acquired during one breath hold. Late gadolinium enhancement (LGE) images were acquired with an inversion-recovery sequence in the AS patients approximately 10 min after the bolus injection of 0.2 mmol/kg gadolinium-based contrast agent.
Diffusion and relaxation parameters were evaluated at the mid-ventricular level. Differences between AS patients and controls were assessed by an unpaired two-tailed t-test. Differences between the patient groups pre- and post-AVR were assessed by a paired two-tailed t-test. A p-value of less than 0.05 was considered statistically significant.
Results
Aortic stenosis vs healthy controlTable 1 shows the baseline characteristics of the AS patient and control groups. The groups were sex-matched, but AS patients were on average 11 years older. In the AS group, the mean pressure gradient across the aortic valve was 44±7 mmHg. Indexed left ventricular mass (LV Mi) and diastolic septal wall thickness (IVSd) were significantly elevated in the AS group which also showed elevated left ventricular ejection fraction (LV EF). Three AS patients exhibited LGE.
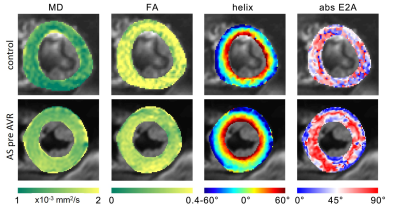
Representative diffusion and structural parameter maps of an AS patient and a control subject are shown in Figure 1. Native T1 mapping was not significantly different between both groups (AS 1049±52 vs controls 1019±44 ms, p=0.19). In contrast, DTI diffusion parameters showed significantly elevated MD (AS 1.63±0.06 vs controls 1.41±0.08 10-3 mm2/s, p<0.001) and reduced FA (AS 0.28±0.02 vs controls 0.35±0.03, p<0.001) in AS patients. Absolute E2A sheet angle (AS 46.8±6.4 vs 33.9±9.5°, p=0.007) as well as HA slope (AS -1.35±0.15 vs controls -1.02±0.15°/%depth, p<0.001) were significantly elevated in AS.
Reversibility following AVR
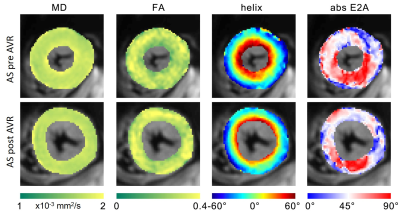
After a median follow-up of 6.3 months post AVR, 8 patients underwent a second CMR (4 surgical AVR, 4 transcatheter AVR). Table 2 summarises the findings in AS patients pre and post AVR. LV Mi was significantly reduced after AVR (p=0.02), while LV EDV, EF and native T1 remain unchanged. CMR DTI showed no significant change in MD, FA and E2A sheet angle after AVR. The HA slope, however, was significantly reduced after AVR (p=0.04) and approached the level of healthy controls (AS post AVR -1.14±0.14 vs controls -1.02±0.15°/%depth, p=0.11). Figure 2 compares CMR diffusion and structural characteristics in an AS patient before and after AVR.
Conclusion
CMR DTI parameters are sensitive to structural alterations within the myocardium caused by AS while native T1 is not. The elevated E2A sheet angle seems to be a common feature of hypertrophic hearts, as it has already been reported in HCM and cardiac amyloidosis patients10,11. In contrast, the HA slope appears to vary significantly with different diseases of hypertrophic phenotype. As shown in this study, HA slope is the only microstructural parameter that returns to normal after AVR. The persistent alteration of the diffusion parameters MD and FA is consistent with previous reports of increased extracellular space in patients after AVR3. The stable elevation of E2A indicates that also the myocardial fibre orientation remains disturbed after normalization of LV afterload. CMR DTI enables identifying subtle but irreversible myocardial damage in response to AS, indicating potential as an imaging biomarker for further optimization of treatment planning.Acknowledgements
This work has been supported by the Swiss National Science Foundation (PZ00P2_174144).References
1. Iung et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003; 24(13):1231-43.
2. Glaser et al. Loss in Life Expectancy after Surgical Aortic Valve Replacement: SWEDEHEART study. J Am Coll Cardiol. 2019; 74(1):26-33.
3. Treibel et al. Reverse Myocardial Remodeling Following Valve Replacement in Patients with Aortic Stenosis. J Am Coll Cardiol. 2018; 71(8):860-71.
4. Sosnovik et al. Diffusion MR tractography of the heart. J Cardiovasc Magn Reson. 2009; 13(11):47.
5. Stoeck et al. Dual-phase cardiac diffusion tensor imaging with strain correction. PLoS One. 2014; 9(9):e107159.
6. Stoeck et al. Second-order motion-compensated spin echo diffusion tensor imaging of the human heart. Magn Reson Med. 2016; 75(4):1669-76.
7. Moulin et al. In-vivo free-breathing DTI & IVIM of the whole human heart using a real-time slice-followed SE-EPI navigator-based sequence: a reproducibility study in healthy volunteers. Magn Reson Med. 2016; 76(1):70-82.
8. von Deuster et al. Spin echo versus stimulated echo diffusion tensor imaging of the in vivo human heart. Magn Reson Med. 2016; 76(3):862-72.
9. Messroghli et al. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004; 52(1):141-6.
10. Gotschy et al. Characterizing cardiac involvement in amyloidosis using cardiovascular magnetic resonance diffusion tensor imaging. J Cardiovasc Magn Reson. 2019; 21(1):56
11. Nielles-Vallespin et al. Assessment of myocardial microstructural dynamics by in vivo diffusion tensor cardiac magnetic resonance. J Am Coll Cardiol. 2017; 69(6):661–76.
Figures

Table 1: Baseline characteristics of the study cohort