1198
Capture the Opening and Closing of Human Aortic Valve Using MRI with Sub-Millisecond Temporal Resolution1Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 2CMRR, University of Illinois at Chicago, Chicago, IL, United States, 3Radiology, University of Illinois at Chicago, Chicago, IL, United States, 4Neurosurgery, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
Stenosis and regurgitation are two common valvular diseases currently diagnosed using echocardiography. Cardiac MR has potential to diagnose these two diseases, however, faces the challenge of inadequate temporal resolution for capturing the rapid opening or closing of aortic valve. Using a variation of a recently proposed technique, coined Sub-millisecond Periodic Event Encoded Dynamic Imaging or SPEEDI (formerly called SMILE), we demonstrated that this process can be visualized using MRI with sub-millisecond temporal resolution. This new capability has improved the accuracy and reliability in studying the dynamics of aortic valve, opening new opportunities to detect stenosis and regurgitation using MRI.
Introduction
Capturing the dynamics of the aortic valve movement is important in detecting valvular disease such as stenosis and regurgitation. However, it has been challenging to visualize this process using MRI due to the rapid movement of the valvular structures and the inadequate temporal resolution of MRI. Typically, the opening and closing happen in less than 50 ms1–3. Even with the highest temporal resolution available for cardiac MRI, only a few time points can be acquired4 during the dynamic process of aortic valve opening and closing. Recently, an ultra-fast MRI technique with sub-millisecond temporal resolution, coined Sub-millisecond Periodic Event Encoded Dynamic Imaging or SPEEDI (formerly called SMILE5,6) was reported, providing an opportunity to capture the opening and closing of aortic valve. In this study, we developed a variation of SPEEDI, which we call epi-SPEEDI, and applied this technique to capture the opening and closing of aortic valve with sub-millisecond temporal resolution.Methods
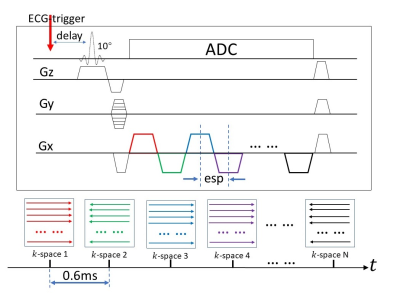
epi-SPEEDI:Built upon SPEEDI, epi-SPEEDI (Fig. 1) extends the FID-based acquisition to an echo-train-based acquisition in a fashion similar to a non-phase-encoded EPI sequence. The epi-SPEEDI sequence is synchronized with an ECG trigger. Each echo in the echo train is positioned in an individual k-space raster, and all echoes in the echo train are spread across a series of time-resolved k-space rasters (k-space 1, k-space 2, … k-space n). This process is repeated with different phase-encoding values until all 2D k-space rasters are adequately sampled. After a 2D Fourier transform, a collection of images can be obtained, providing a time-resolved description of the dynamic process with a temporal resolution determined by echo spacing (esp). Using ramp sampling, a sub-millisecond temporal resolution can be achieved on commercial human scanners. Compared to SPEEDI, epi-SPEEDI substantially shortens the overall scan time at the expense of reduced temporal resolution. Although our demonstration was limited to 2D, the same concept can be expanded to 3D with an additional phase-encoded direction.
Multi-phase and “Dovetail” Acquisition Strategies:
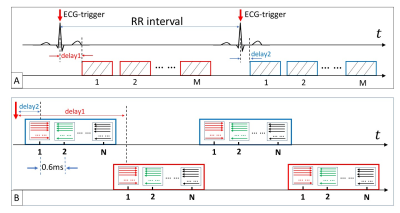
In order to cover the entire RR interval, two acquisition strategies were used in this study: multi-phase and “dovetail” (Fig. 2). A primary multi-phase approach was used to cover a longer time span, where each acquisition block was repeated immediately after the preceding acquisition block was finished (Fig. 2A). Due to the RF pulse and crusher gradients, a blank time was present between the acquisition blocks. To fill the blank time between acquisition blocks, a secondary “dovetail” acquisition strategy was employed (Fig. 2B), where two different trigger delays were used (red and blue). The red acquisition blocks “dovetailed” the blue acquisition blocks to fill the blank time. The difference of two trigger delays should be no longer than the acquisition blocks.
Data Acquisition and Analysis:
An epi-SPEEDI sequence (Fig. 1) was implemented on a 3T GE MR750 scanner. With IRB approval, cardiac MR images were acquired from healthy human subjects. Short-axis view was selected for imaging the aortic valve to capture all the three cuspids. The key sequence parameters were: slice thickness=8mm, FOV=22cm×22cm, matrix=118×118, esp=0.6ms, trigger delay=12ms/22ms, flip angel=10º, acquisition time=160 heart beats. The acquired k-space data were reconstructed offline using a customized Matlab program. The reconstructed images were then realigned according to their acquisition time based on the acquisition strategies described in Fig. 2. The anatomical area of aortic valve (AOA) was calculated for each frame to monitor its dynamic change.
Results
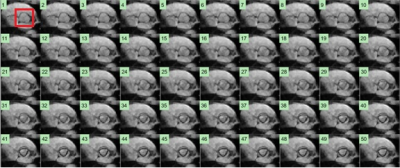
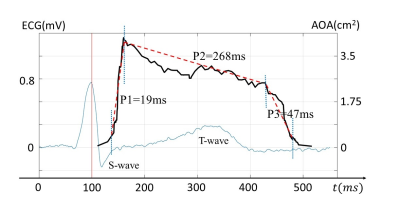
Figures 3 and 4 show a set of images with a temporal resolution of 0.6ms during the opening and closing of aortic valve, respectively. The dynamics of the opening and closing process of aortic valve were clearly observed. A plot of the anatomic area of aortic orifice in the time course quantitively shows the opening and closing process of aortic valve as illustrated in Fig. 5. The opening process started immediately after the QRS-complex, whereas the closing process ended after the T-wave, both of which matched well with the ECG waveform. The three phases of the entire process of aortic valve opening and closing were also well identified in Fig. 5: a rapid opening phase (P1=19ms), a slowly closing phase (P2=268ms), and a rapid closing phase (P3=47ms).Discussion and Conclusion
We were able to capture the dynamics of the opening and closing of human aortic valve with a temporal resolution of 0.6ms using an epi-SPEEDI sequence. This study extends the capability of SPEEDI from capturing ultra-fast physical processes reported previously to an ultra-fast physiologic process – aortic valve opening and closing. The three phases during the opening and closing process of aortic valve have been demonstrated in previous studies using echocardiography on the human heart1,7, as well as on mouse heart using MRI2. In the animal studies, the inferior temporal resolution to what was achieved in our study can lead to overestimation of the rapidly opening process and underestimation of the slowly closing process. Using epi-SPEEDI with sub-millisecond temporal resolution, the dynamic process of aortic valve opening and closing can be more accurately and reliably visualized. Our future studies will focus on applying epi-SPEEDI to patients with aortic valve dysfunctions.Acknowledgements
This work was supported in part by NIH 1S10RR028898. We thank Dr. Afshin Farzaneh-Far for helpful discussions.References
1. Leyh, R. G., Schmidtke, C., Sievers, H.-H. and Yacoub, M. H. Opening and Closing Characteristics of the Aortic Valve After Different Types of Valve-Preserving Surgery. Circulation 100, 2153–2160 (1999).
2. Weisell, J. et al. Characterizing valve dynamics in mice by high‐resolution cine‐MRI. NMR Biomed. e4108 (2019) doi:10.1002/nbm.4108.
3. Thubrikar, M. J., Heckman, J. L. and Nolan, S. P. High speed cine-radiographic study of aortic valve leaflet motion. J. Heart Valve Dis. 2, 653–661 (1993).
4. Krishnamurthy, R., Pednekar, A., Cheong, B. and Muthupillai, R. High temporal resolution SSFP cine MRI for estimation of left ventricular diastolic parameters. J. Magn. Reson. Imaging 31, 872–880 (2010).
5. Zhong, Z., Karaman, M. M. and Zhou, X. J. MRI with Sub-Millisecond Temporal Resolution: An Example Employing Spatially Resolved Eddy Current Characterization. in ISMRM2019 P0247.
6. Zhong, Z., Karaman, M. M., Claiborne, T. and Zhou, X. J. Capturing Time-Dependent Electric Currents Using MRI with A Sub-Millisecond Temporal Resolution. in ISMRM2019 P4574.
7. Handke, M. et al. In vivo analysis of aortic valve dynamics by transesophageal 3-dimensional echocardiography with high temporal resolution. J. Thorac. Cardiovasc. Surg. 125, 1412–1419 (2003).
Figures