1197
Chronic Myocardial Infarcts with Iron Deposits Exhibit Lower Rest Perfusion and Elevated Nitric Oxide Synthase Activity
Eric Johnson1,2, Anand Nair2, Ivan Cokic1,2, Hsin-Yung Yang2, Andreas Kumar3, and Rohan Dharmakumar1,2
1UCLA, Los Angeles, CA, United States, 2Cedars Sinai, Los Angeles, CA, United States, 3Northern Ontario School of Medicine, Thunder Bay, ON, Canada
1UCLA, Los Angeles, CA, United States, 2Cedars Sinai, Los Angeles, CA, United States, 3Northern Ontario School of Medicine, Thunder Bay, ON, Canada
Synopsis
Hemorrhagic myocardial infarction (hMI) patients are predisposed to adverse outcomes in the chronic stage of MI, yet physiological underpinnings contributing to this observation are not well understood. We hypothesized that hMI areas containing iron deposits would negatively impact endothelial function and tested our hypothesis by evaluating perfusion defects in patients and dogs with a history of hMI; with histological staining for iron, endothelial cells and nitric oxide synthase (NOS) in excised myocardial sections of dogs with chronic MI. Hemorrhagic subjects had significantly reduced perfusion and markedly elevated NOS activity.
Introduction
Hemorrhagic myocardial infarction (hMI) patients are predisposed to adverse outcomes in the chronic stage of MI, yet physiological underpinnings contributing to this observation are not well understood. We investigated the connection between resting perfusion, vascular characteristics and iron deposition in infarct territories with cardiac MRI and histology. Given that iron is known to scavenge nitric oxide (NO), we hypothesized that hMI areas containing iron deposits would negatively impact endothelial function in these same regions. We tested our hypothesis by evaluating perfusion defects in patients and dogs with a history of hMI; and through histological staining for iron, endothelial cells and nitric oxide synthase (NOS) in excised myocardial sections of dogs with chronic hMI.Methods
Patient Studies: Reperfused infarction (MI) patients (n=14) were studied with MRI at 6 months post reperfusion. T2*-w, first-pass perfusion (FPP) at rest and LGE images were acquired in a clinical 1.5T system. Rest perfusion scans matched to T2* and LGE scans were acquired and analyzed in CVI42. Imaging slices positive for MI were categorized as hemorrhagic (hMI+) or non-hemorrhagic (hMI-) based on T2* images. MI and iron-rich regions were segmented using mean+5SD and mean-2SD criteria, respectively. For each imaging slice, Relative Perfusion Index (RPI) was calculated as a ratio of signal intensity upslope in MI zone to remote zone. T-test was used to study the perfusion difference between hMI+ and hMI- territories. Statistical significance was set at p<0.05.Animal Studies: Dogs (n=22) were subjected to ischemia reperfusion of the LAD and followed for 8 weeks. At 8 weeks post MI, T2*-w, FPP and LGE MRI scans were performed in a clinical 3T system. Data was analyzed as described in the patient study. Excised hearts were fixed in formalin and embedded in paraffin for histological sectioning and staining. Apical, MI-containing sections were stained for Prussian Blue (iron), CD31 (endothelial cells) and eNOS/iNOS (endothelial/inducible NOS). Stained slides were digitized and processed in Definiens software. An in-house Matlab script was used to register the Prussian blue slides with CD31 counterparts to facilitate regional analyses.
Results
Patients with hemorrhagic infarctions (hMI+, characterized by iron deposits), had decreased RPI in infarct zones relative to patients with non-hemorrhagic (hMI-) infarcts (0.72±0.11 vs. 0.83±0.08 respectively, p=0.003), see Fig. 1. A corroboratory reduction in RPI was also observed in the canine cohort (hMI+ (0.62 ± 0.16) vs. hMI- (0.85 ± 0.14)) at 8 weeks post-MI; see Fig. 2. Preliminary evidence shows that endothelial NOS (eNOS) is upregulated in hMI+ animals compared to hMI- animals (Fig. 3D, E); and that the number of vessels within the scarred myocardium and iron deposits are positively correlated (Fig. 3A, B, C). Similar findings in iNOS-stained slides were found in that hMI+ animals had increased iNOS activity compared to hMI- animals.Discussion
Major adverse cardiac events (MACE) are a major hurdle in the prognosis of hemorrhagic myocardial infarctions and elucidating the pathological relationships alongside MRI markers of hemorrhagic MI may help to uncover unique therapeutic targets for the treatment of these severe types of infarctions. This study provides a significant step supporting how hemorrhagic MIs could promote adverse outcomes. Accordingly, it lays the groundwork for further studies in this field.Conclusion
MRI findings show that hMI results in rest perfusion defects within the scarred myocardium. Histological findings from animals support the notion that iron deposition within the MI zones may impact nitric oxide synthase activity, which is a key mediator of vascular flow. These findings provide early insight into the causal relationships between chronic iron deposits and negative outcomes observed in patients with hMI.Acknowledgements
No acknowledgement found.References
No reference found.Figures
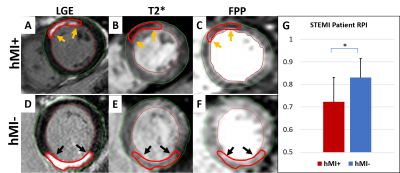
Fig. 1. Patients. A: LGE showing an anterior-wall MI (orange arrows). B: Slice-matched T2*-weighted image showing
significant hypo-enhancement in MI zone. C: First-pass perfusion (FPP)
frame showing significant perfusion defect in hMI+ zone at peak myocardial
enhancement. D: LGE slice showing the large inferior-wall infarction
(black arrows). E: Slice-matched T2*-weighted image lacking hypo-enhancement in the infarct zone (black arrows). F: FPP frame
without the visible perfusion defect seen in hMI+
case (C). G: Mean RPI at rest in the
chronic MI zones of hMI+ vs. hMI- patients.
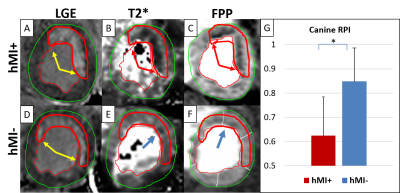
Fig. 2. Canines A: LGE slice in hMI+ animal (yellow arrows). B: Slice-matched T2*-weighted image showing
significant hypo-enhancement in MI zone. C: FPP frame showing significant perfusion defect in hMI+ zone at peak myocardial
enhancement. D: LGE slice showing a large anterior infarction (yellow
arrows). E: Slice-matched T2*-weighted image with minimal
hypo-enhancement in the infarct zone (blue arrow). F: FPP frame without
the visible perfusion defect at peak myocardial enhancement seen in IMH+ case
(C). G:Mean RPI at rest in the chronic MI zones of hMI+ vs. hMI- animals.
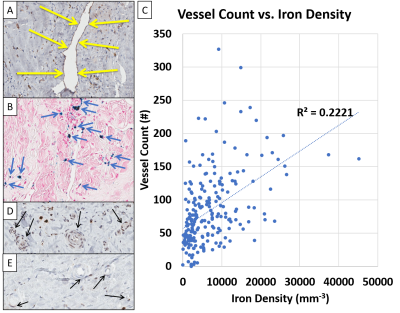
Fig.
3. Relation between Iron Density, Vessel Density and eNOS Activity. A. CD31 stain for endothelial cells highlighting a
myocardial vessel (yellow arrows) in the infarct zone of an IMH+ animal. B.
Prussian blue stain displaying iron (blue arrows) in the same region of the
infarct zone as in A. C. Positive correlation between vessel counts and
iron density in the infarct zone. D. eNOS staining showed evidence of
eNOS in hMI+ animals. E. hMI-
animals had markedly lower eNOS staining in the infarct region.