1188
Evaluation of APT imaging in parotid glands and strategy in clinical usage1Peking Union Medical College Hospital, Beijing, China, 2Philips HealthCare, Beijing, China, Beijing, China
Synopsis
This study was to prospectively evaluate APT imaging for the parotid glands and lesions. 32 patients, confirmed cancer in parotid glands, underwent 3D TSE APTw imaging. Scores for integrity and for hyperintensity artifacts of both tumor lesions and normal parotid glands were evaluated. Tumor lesions had better integrity score than normal parotid glands. Scores for hyperintensity artifacts in APTw images showed no significant difference between tumor lesions and normal parotid glands. Most APTw images of parotid glands lesions were scored with good integrity and had acceptable image quality, while challenges still exist in some cases.
Background and Purpose
MRI plays a key role in discriminating benign and malignant parotid tumors, and the correct diagnosis is crucial for choosing optimal surgical strategy or radiation therapy plan[1]. Hence advanced MRI techniques beyond morphological imaging has been investigated to provide biomarkers for tumor characterization[2]. Amide proton transfer (APT) imaging[3], non-contrast molecular imaging method, has been proven an effective tool for tumor detection and characterization in the brain, and there has been preliminary APT findings in the breast, prostate, and chest[4]. Hereby we investigated the feasibility of APTw imaging in application of head and neck, based on image quality and the differentiation of malignant tumors, benign lesions and normal tissues. The aim of this study was to prospectively evaluate the utility of APT imaging in the parotid glands and lesions.Material and methods
This prospective study was approved by the institutional review board. Between October 2017 and December 2018, 32 patients, who were confirmed tumors in parotid glands, underwent three-dimensional (3D) turbo-spin-echo (TSE) APTw MR imaging on a 3T MR scanner (Ingenia CX, Philips Healthcare, the Netherlands) equipped with dual RF transmit coils and a 32-channel head-coil for image acquisition. The commercially available 3D APT sequence embedded a 3D TSE readout and acquired a z-spectrum with the saturation targeting at the following frequencies: 2.7 ppm, 3.5 ppm, 4.3 ppm, and -1560 ppm (regarding to the water proton frequency). Images were acquired 3 times at the +3.5 ppm with an echo shift of 0.5 ms, based on which B0 field map was calculated for z-spectrum correction. Two transmission channels were turned on alternatively to produce 4 saturation pulses in consequence, each 500 ms in duration with an amplitude of 2 μT, to achieve sufficient saturation of 2s long. The APTw value (in percentage) was calculated as the signal difference between the 3.5 ppm images, normalized to the image with the saturation pulse at -1560 ppm. Integrity of tumor lesions or normal parotid glands was evaluated based on a 4-point Likert scale (3=all the lesion or normal parotid gland was displayed; 0=lesion or normal parotid gland was not displayed at all). Image quality of APTw image on tumor lesions, regarding to hyperintensity artifacts diffused from surrounding tissue, was evaluated based on a 4-point Likert scale (4=excellent, 1=poor). Two radiologists with 10 and 3 years of head and neck experience and knowledge of the tumor histologic features independently reviewed all images. Regions of interests (ROI) of tumor lesions or normal parotid gland were manually drawn on the APTW images when integrity score was more than 1; and the APT values were recorded in each ROI. The results of tumor lesions and the contralateral side normal parotid glands of the same patients were all analyzed. Then (1)integrity scores, (2)the image quality scores relating to hyperintensity artifacts, and (3)APTw value were compared between tumor lesions and normal parotid glands.Results
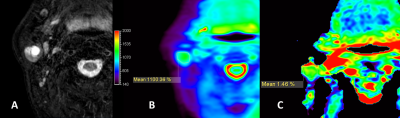
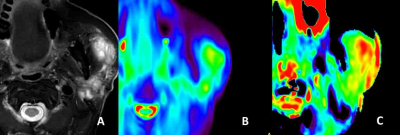
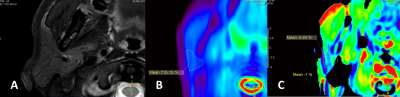
31 out of 32 lesions (96.9%, 31/32) were considered 3 score in integrity evaluation, except one lesion was poorly imaged as scored 0. Among the 26 normal parotid glands, 3 (11.5%, 3/26) were evaluated as 2 score, and 19 (73%, 19/26) were evaluated as 3 score. There was a significant difference between tumor lesions and normal parotid gland (P=0.037) in integrity score. Hence the integrity evaluation could serve for quality control in APTw image reading. After lesions or normal parotid glands with integrity score 0 were excluded, 12.9% (4/31) and 48.4% (15/31) of lesions, and 4.2 % (1/24), and 50,0% (12/24) of normal parotid glands were considered as 3 and 4 score respectively when evaluating the hyperintensity artifacts. There was no significant difference between tumor lesions and normal parotid glands (P=0.787). Examples of integrity and hyperintensity artifacts were shown in Fig 1-3. APTw values of tumor lesions were measured [ 2.33%±1.55 (range, 0.19%-6.06% )]; and normal parotid glands were also measured[2.23%±2.12 (range, -0.96%-6.28% )], in which we identified the hyperintensity artifacts diffused from surrounding tissue and raised the average.Discussion
The present study demonstrates the first attempt of 3D TSE APTw MR imaging for parotid gland tumors. The saturation RF pulses of the APT sequence lasted for two seconds, and the resulting APTw signal value corresponds to this particular B1 setting (labelling time and strength). Some reading challenges presented in APTw images due to the low-pass filtering effect. The images have a low-resolution visualization after filtering, then hyperintensity artifacts from low- fidelity APTw values near bone and air diffused to parotid glands, and sometimes triggering false positives. However, our results found the integrity evaluation was useful in screening for false positive and could serve for quality control. A higher-resolution APT imaging could be developed and potentially further improve the diagnosis of small lesions in parotid gland.Conclusion
Our preliminary study of parotid tumors APT imaging demonstrated that 3D APTw can be used to detect parotid tumors and normal parotid glands. Most of tumor lesion of parotid glands were displayed in integrity evaluation and had acceptable image quality in APTw images.Acknowledgements
We thank the supporting of department of stomatology in Peking Union Medical College Hospital for patients visiting and follow-up.References
[1] Yu L, Li C, Luo X, et al. Differentiation of Malignant and Benign Head and Neck Tumors with Amide Proton Transfer-Weighted MR Imaging. Mol Imaging Biol. 2018 .
[2] Ohno Y, Yui M, Koyama H, et al. Chemical Exchange Saturation Transfer MR Imaging: Preliminary Results for Differentiation of Malignant and Benign Thoracic Lesions. Radiology. 2016. 279(2): 578-89.
[3] Zhou J, Zhu H, Lim M, et al. Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging. 2013. 38(5): 1119-28.
[4] Takayama Y, Nishie A, Sugimoto M, et al. Amide proton transfer (APT) magnetic resonance imaging of prostate cancer: comparison with Gleason scores. MAGMA. 2016. 29(4): 671-9.
Figures