1180
Is recovery from whiplash influenced by macromolecular changes in spinal cord white matter?1Biomedical Engineering, Northwestern University, Chicago, IL, United States, 2Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, IL, United States, 3Northern Sydney Local Health District & Faculty of Health Sciences, The University of Sydney, Sydney, Australia, 4Preventive Medicine, Northwestern University, Chicago, IL, United States, 5Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Palo Alto, CA, United States, 6Radiology, Northwestern University, Chicago, IL, United States
Synopsis
Whiplash injuries are the most common outcome from non-fatal motor vehicle collisions, affecting nearly four million people in the United States each year. The purpose of this cross-sectional study was to investigate the macromolecular environment of cervical spinal cord white matter in participants with persistent whiplash. This investigation of 76 individuals demonstrated changes in cervical white matter integrity following whiplash injuries using magnetization transfer imaging. Significant differences in the magnetization transfer ratio homogeneity of large cervical white matter tracts were observed in females with poor clinical outcome, indicating a spinal cord insult may contribute to chronic pain after whiplash injury.
Introduction
Whiplash injuries are the most common outcome from non-fatal motor vehicle collisions (MVC), costly, and have a high rate of transition to chronicity, affecting nearly four million people in the United States each year. (1,2) nearly a quarter of those injured (up to one million) will present with, and report a wide variety of, signs and symptoms characterized by neck pain, headache, widespread sensory hypersensitivity, changes in motor performance, cognitive interference, changes in bodily muscle composition, sensorimotor integration deficits, and psychopathology. (3-10) Unfortunately, standard imaging protocols have not consistently revealed relevant lesions in patients who transit from acute to chronic whiplash associated disorders (WAD). (11-12)Given the varied mechanics of a typical motor vehicle crash (e.g. rear-end, side, or frontal), a subtle insult involving the cervical spinal cord is feasible, but likely occult with conventional imaging. (13-15) Magnetization transfer (MT) imaging could provide an early and accurate quantification of an insult involving the cord following MVC. The purpose of this cross-sectional study was to investigate whether MT imaging was able to identify those participants injured from an MVC whose clinical signs and symptoms were consistent with an insult to cervical spinal cord white matter.
Methods
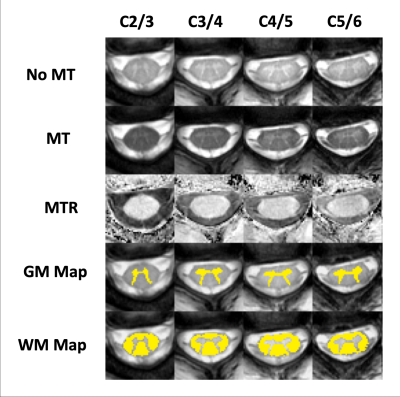
Participants were selected from a completed prospective study of 76 injured participants who presented with WAD Grade II, via Quebec Task Force classification, to an emergency department following an MVC. Clinical outcome was determined by Neck Disability Index (NDI) score.(16) MT images were collected one year after MVC and were parallel to the cervical intervertebral disks between C2 and C6. Figure 1 illustrates spinal cord white and grey matter segmentations, performed using convolutional neural network functions from the Spinal Cord Toolbox (SCT).(17) As shown in Figure 2, Magnetization transfer ratios (MTR) were measured in the bilateral corticospinal tracts, combined spinothalamic and spinoreticular tracts, and cuneate and gracile fasciculi using the PAM50 atlas in the SCT.(18) The homogeneity of the MTR response (MTRh) was measured across the large white matter tracts at each given slice using the following equation:$$MTRh = \left(\frac{1}{\sqrt{N_A}}\right)\frac{\sqrt{\left(\frac{1}{\sqrt{N_A-1}}\right)\sum\left(X_A-\bar{X}\right)^2}}{\frac{1}{N_A}\sum X_A}$$
where $$$X_A$$$ represents the regional MTR values in the ventromedial, dorsal and dorsolateral white matter aspects of the cord, $$$\bar{X}$$$ is the average of the MTR values and $$$N_A$$$ is determined by the number of regions measured (8 in this study). MTRh ranges from completely homogenous, at 0, and increases towards 1 as inhomogeneities in the measured quantities increase.
A generalized linear mixed model was created, adjusting for repeated measures within subject using compound symmetry, and multiple imputation methods (5 sets) to impute missing data. Least Square means for MTRh were calculated adjusting for BMI, age, race, and cervical level.
Results
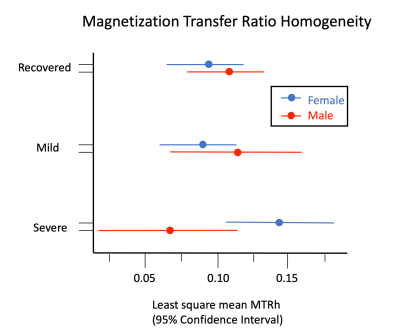
There is a significant interaction between recovery status and sex (p=0.015). Least square means for the female and male recovered groups were: 0.094 and 0.111; mild groups: 0.087 and 0.112; severe: 0.140 and 0.067. Least square mean MTRh values for females with severe clinical outcome were significantly difference from recovered females (p=0.023), mild continuing symptoms (p=0.005), and males with severe outcome (0.010).Discussion
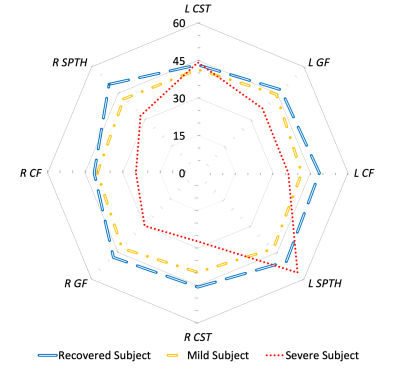
Significant differences in MTRh were observed between the severe whiplash female group versus the other clinical outcomes and sex, as shown in Figure 3. These findings support recent literature and clinical observations of potential insults to the spinal cord in those with severe WAD.(15,19) Additionally, such findings may point to secondary signs of an insult to the cord or the surrounding peripheral tissues. Altered cord glucose metabolism due to direct neuronal insult or the presence of a glutamatergic inflammatory process from a peripheral injury could also be occurring in those with persistent WAD.(20) Identifying significant group differences when controlling for outcome and sex supports long-standing acceptance that females are at greater risk of chronicity as compared to males.(21) Overall, these findings identify an important subgroup of patients with WAD who have signs and symptoms, but no standard protocol radiological features, that are strikingly similar to the known pathophysiology of mild incomplete spinal cord injury.We have proposed a subject-normalized metric (MTRh) to identify changes in MT signal that may be related to spinal cord white matter demyelination. Additionally, due to inherent differences in body composition and size of participants, differing inhomogeneities of the magnetic field within and between scans, and differences in transmitter and receiver coil loads, MT signals are difficult to reproduce on a subject-specific level. The MTRh metric avoids these pitfalls. Figure 3 is a visual representation of MTRh, and illustrates stark differences between the three clinical outcome groups. Normalized methods are critically important when making direct subject-to-subject comparisons of subtle MTR variations.
Conclusions
This investigation demonstrates the utility of MT imaging of the spinal cord to identify tract specific and regional changes in spinal cord white matter integrity following whiplash injuries. Significant differences in MTRh between sexes were observed. These findings of increased heterogeneity levels (higher MTRh) for females with severe clinical outcomes at 3 months support many large-scale clinical studies detailing poorer recovery in females when compared to males. This work supports the hypothesis that damage to the cervical spinal cord may underlie the transition to chronic WAD in some, not all, injuredAcknowledgements
No acknowledgement found.References
1. Naumann RB, Dellinger AM, Zaloshnja E, Lawrence B, MillerTR. Incidence and Total Lifetime Costs of Motor Vehicle-Related Fatal and Nonfatal Injury by Road User Type, United States. Traffic Injury Prevention. 2010;11(4):353-60.
2. Carroll LJ, Holm LW, Hogg-Johnson S, Côtè P, Cassidy JD, Haldeman S, et al. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Journal of manipulative and physiological therapeutics. 2009;32(2):S97-S107.
3. Kasch H, Qerama E, Bach FW, Jensen TS. Reduced cold pressor pain tolerance in non-recovered whiplash patients: a 1-year prospective study. Eur J Pain. 2005;9(5):561-9.
4. Treleaven J, Jull G, Sterling M. Dizziness and unsteadiness following whiplash injury: characteristic features and relationship with cervical joint position error. J Rehabil Med. 2003;35(1):36-43.
5. Treleaven J JG, LowChoy N. Smooth-pursuit neck torsion test in whiplash-associated disorders: relationship to self-reports of neck pain and disability, dizziness and anxiety. J Rehabil Med. 2004;36:1-5.
6. Woodhouse A, Liljeback P, Vasseljen O. Reduced head steadiness in whiplash compared with non-traumatic neck pain. J Rehabil Med. 2010;42(1):35-41.
7. Schomacher J, Farina D, Lindstroem R, Falla D. Chronic trauma-induced neck pain impairs the neural control of the deep semispinalis cervicis muscle. Clin Neurophysiol. 2012;123(7):1403-8.
8. Dufton JA, Bruni SG, Kopec JA, Cassidy JD, Quon J. Delayed recovery in patients with whiplash-associated disorders. Injury. 2012;43(7):1141-7.
9. Abbott R, Pedler A, Sterling M, Hides J, Murphey T, Hoggarth M, et al. The geography of fatty infiltrates within the cervical multifidus and semispinalis cervicis in individuals with chronic whiplash-associated disorders. J Orthop Sport Phys. 2015;45(4):281-8.
10. Elliott J, Pedler A, Kenardy J, Galloway G, Jull G, Sterling M. The temporal development of fatty infiltrates in the neck muscles following whiplash injury: an association with pain and posttraumatic stress. PloS one. 2011;6(6):e21194.
11. Mathen R, Inaba K, Munera F, Teixeira PG, Rivas L, McKenney M, et al. Prospective evaluation of multislice computed tomography versus plain radiographic cervical spine clearance in trauma patients. J Trauma. 2007;62(6):1427-31.
12. Platzer P, Hauswirth N, Jaindl M, Chatwani S, Vecsei V, Gaebler C. Delayed or missed diagnosis of cervical spine injuries. Journal of Trauma and Acute Care Surgery. 2006;61(1):150-5.
13. Guez M, Hildingsson C, Rosengren L, Karlsson K, Toolanen G. Nervous tissue damage markers in cerebrospinal fluid after cervical spine injuries and whiplash trauma. J Neurotrauma. 2003;20(9):853-8.
14. Svensson MY, Aldman B, Bostrom O, Davidsson J, Hansson HA, Lovsund P, et al. [Nerve cell damages in whiplash injuries. Animal experimental studies]. Orthopade. 1998;27(12):820-6.
15. Elliott JM, Dewald J, Hornby TG, Walton DM, Parrish TB. Mechanisms underlying chronic whiplash: contributions from an incomplete spinal cord injury? Pain medicine. 2014;15(11):1938-44.
16. Ritchie C, Sterling M. Recovery Pathways and Prognosis After Whiplash Injury. J Orthop Sports Phys Ther. 2016;46(10):851-61.
17. De Leener B, Lévy S, Dupont SM, Fonov VS, Stikov N, Collins DL, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017;145:24-43.
18. De Leener B, Fonov VS, Collins DL, Callot V, Stikov N, Cohen-Adad J. PAM50: unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage. 2018;165:170-9.
19. Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Frontiers in neurology. 2019;10:282.
20. Smith AC, Parrish TB, Hoggarth MA, McPherson JG, Tysseling VM, Wasielewski M, et al. Potential associations between chronic whiplash and incomplete spinal cord injury. Spinal cord series and cases. 2015;1:15024.
21. Kamper, S.J., et al., Course and prognostic factors of whiplash: a systematic review and meta-analysis. Pain, 2008. 138(3): p. 617-29.
Figures