1179
Inhomogeneous Magnetization Transfer and DBSI detect downstream white matter damage in post-mortem human cervical spinal cord injury1International Collaboration on Repair Discoveries, Vancouver, BC, Canada, 2Physics & Astronomy, University of British Columbia, Vancouver, BC, Canada, 3Radiology, University of British Columbia, Vancouver, BC, Canada, 4UBC MRI Research Centre, Vancouver, BC, Canada, 5Carson Graham Secondary School, Vancouver, BC, Canada, 6Pathology & Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada, 7Vancouver General Hospital, Vancouver, BC, Canada, 8Medicine, University of British Columbia, Vancouver, BC, Canada, 9Vancouver Spine Surgery Institute, Vancouver, BC, Canada, 10Orthopaedics, University of British Columbia, Vancouver, BC, Canada
Synopsis
Spinal cord injuries are heterogeneous, with complex microstructure which changes over time. We used 7T Diffusion Tensor Imaging (DTI), Diffusion Basis Spectrum Imaging (DBSI) and inhomogeneous Magnetization Transfer (ihMT) to investigate microstructural damage in post-mortem human spinal cord injury tissue. We measured sharp decreases in DTI fractional anisotropy and DBSI fiber fraction at the injury epicentre of the three cords with the most severe injuries. We found evidence for downstream demyelination (ihMT) and axonal loss (DTI FA, DBSI fiber fraction) in the two cords with the longest injury-death interval suggesting a time-frame for the detection of Wallerian degeneration by MRI.
Introduction
Spinal cord injury (SCI) is heterogeneous in severity, neurological level and injury mechanism. Clinical trials are increasingly focussed on providing targeted therapies for SCI patient subpopulations, stratified by specific injury metrics. This relies on accurate information about the complex injury microstructure and how it is changing over time.1 Wallerian degeneration begins after the primary injury and continues for years, comprising disintegration of downstream axons and associated myelin sheaths after connection with the neuronal cell body is severed.2,3 Oligodendrocyte apoptosis starts immediately4 followed by slow myelin loss over years.5 Conventional and diffusion tensor imaging (DTI) have detected Wallerian degeneration with T2 signal changes occurring in the 1-3 months post-injury6 and DTI abnormalities reported in the chronic stage7. Several other quantitative MRI techniques may help to measure tissue microstructure and differentiate injury processes.Diffusion Basis Spectrum Imaging (DBSI) is a model developed to distinguish aspects of complex tissue pathology which are inseparable using DTI analysis (DTI fits only one diffusion tensor to each voxel, producing fractional anisotropy (FA), radial, axial and mean diffusivity (RD, AD, MD) maps). DBSI involves a complex data-fitting process, separating signal into anisotropic components (fiber fraction) and isotropic component fractions: low diffusivity restricted (intracellular water), higher diffusivity hindered (extracellular water), and free water.8–10
Inhomogeneous Magnetization Transfer (ihMT) is a novel myelin-sensitive technique which produces a regional map of dipolar order.11,12 Recent advances in ihMT acquisition have included reducing the sensitivity to T1 and B1 inhomogeneities using the inverse of the saturated signal (ihMTRex),13 and increasing the spacing between pre-pulses to increase myelin specificity, which has a long dipolar relaxation time (T1D filtering).14,15
Objective: Investigate advanced MRI biomarkers for tissue degeneration after SCI, and assess the time course of MRI-detected Wallerian degeneration using post-mortem human spinal cord tissue.
Methods
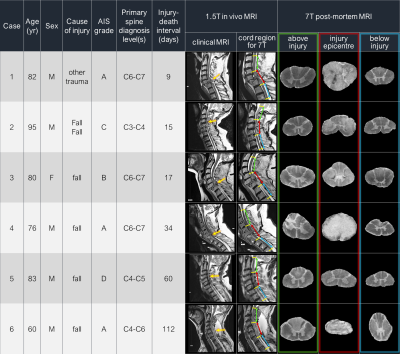
Acquisition: Six full-length spinal cords from patients with acute/sub-acute cervical SCI were donated to the International SCI Biobank (Figure 1). For each cord, 3 formalin-fixed 4.5cm segments, centred at injury epicentre, were imaged at 7T (Bruker Biospec, 35mm inner-diameter quadrature volume coil, room temperature) to obtain: (1) Anatomical (RARE, slices=45, resolution=0.1x0.1x1mm3) (2) Diffusion (multi-shell 3D diffusion-weighted SE EPI, TR/TE=250/41.21ms, resolution=0.15x0.15x1mm3, six b=0 scans, 5 shells with b=500,1000,2000,3500,5000,7000s/mm2 and 6,15,24,42,60,80 directions respectively, distributed uniformly by a Spherical Code optimization algorithm16) (3) ihMT with T1D-filtering (segmented 3D-FLASH, TR/TE=100/2.888ms, resolution=0.36x0.36x1.5mm3, 12 alternating-frequency 3ms pre-pulses with 0.3ms separation, ±8kHz from water resonance).MRI analysis: Non-local mean denoising was used to pre-process all data17. Diffusion data were susceptibility and eddy current corrected (FSL ‘top up’,‘eddy’18,19) and fit with DTI and DBSI8. ihMTRex13 maps were created in MatLab (in-house procedure). Anatomical images were manually segmented into white matter (WM) and grey matter masks, which were registered slice-wise to a histological spinal cord atlas using a Coherent Point Drift algorithm.20,21 Metric values from each WM tract were extracted for every slice.
Results
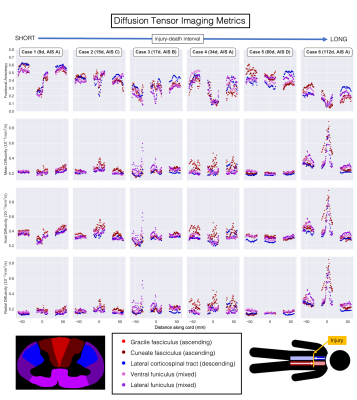
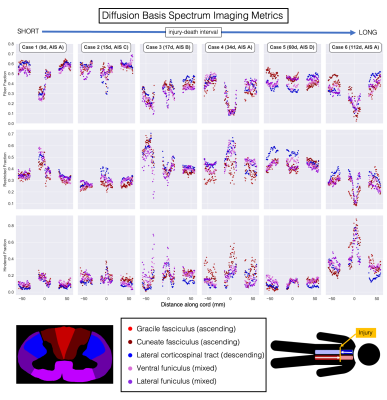
DTI (Figure 2): Excluding Case 5, FA decreased with longer injury-death interval. FA was sharply decreased at the epicentre in the three cords with the most severe AIS injury score (A) (Cases 1,4,6). A downstream (descending tracts below the injury, ascending tracts above) decrease in FA was seen in the two cords with the longest injury-death interval (Cases 5,6). MD was much higher in Case 6.DBSI (Figure 3): Fiber fraction was increased in the two cords with short injury-death interval (Case 1,2). Fiber fraction showed downstream decreases in Cases 5,6 similar to FA. Restricted fraction was highest in Cases 3,4,5. Hindered fraction was much higher in Case 6, suggesting the increased DTI MD is being assigned to the extracellular DBSI component.
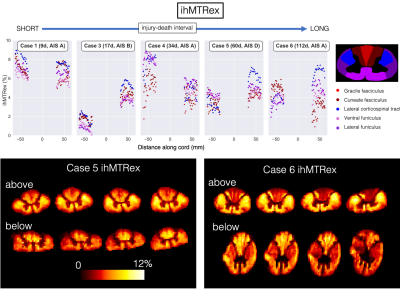
ihMT (Figure 4): ihMTRex was lower in the downstream areas of Cases 5 and 6. Representative imaging slices show lower ihMTRex in dorsal (ascending sensory) WM above the epicentre and in lateral corticospinal tracts below the epicentre.
Discussion
In this study we had a unique opportunity to conduct post-mortem imaging of human spinal cords after traumatic injury. As expected, cords with a shorter injury-death interval had higher FA and fiber fraction, as there was less time for axons to degenerate thereby increase diffusion isotropy. The sharp decrease in FA at the injury epicentre in cases with a complete injury is consistent with severe focal WM damage (anatomical images in Figure 1). Lower fiber fraction and FA in downstream areas for Cases 5 and 6 may be measuring decreased WM integrity and axonal loss due to Wallerian degeneration, supporting previous in vivo DTI findings in chronic SCI.7 ihMTRex in Cases 5 and 6 provides evidence of downstream myelin loss. Combined with ongoing histological analysis (Figure 5), our results may give a time interval for measuring slow demyelination and axon degeneration with ihMT and diffusion techniques.Conclusion
A sharp FA decrease was seen at the injury epicentre in patients with no motor/sensory function below the injury, which was not observed in patients with less severe injuries. ihMT and diffusion imaging metrics demonstrated downstream WM damage in SCI patients with a long (60-112 day) injury-death interval which was not found in cases with a shorter (9-34 day) injury-death interval, establishing a time-frame for the detection of Wallerian degeneration with these MRI methods.Acknowledgements
We would like to thank the patients and families for tissue donation to the International Spinal Cord Injury Biobank. Funding for the Biobank and this study was provided by the Blusson Integrated Cures Partnership (BICP), VGH and UBC Hospital Foundation and the Rick Hansen Foundation, an International Collaboration on Repair Discoveries (ICORD) seed grant and NSERC.References
1. Ahuja, C. S. et al. Traumatic Spinal Cord Injury—Repair and Regeneration. Neurosurgery 80, S9–S22 (2017).
2. Waller, A. V. & Owen, R. XX. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. Lond. 140, 423–429 (1850).
3. Becerra, J. L. et al. MR-pathologic comparisons of wallerian degeneration in spinal cord injury. Am. J. Neuroradiol. 16, 125–133 (1995).
4. Emery, E. et al. Apoptosis after traumatic human spinal cord injury. J. Neurosurg. 89, 911–920 (1998).
5. Buss, A. et al. Sequential loss of myelin proteins during Wallerian degeneration in the human spinal cord. Brain 128, 356–364 (2005).
6. Kuhn, M. J. et al. Wallerian degeneration after cerebral infarction: evaluation with sequential MR imaging. Radiology 172, 179–182 (1989).
7. Cohen-Adad, J. et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. NeuroImage 55, 1024–1033 (2011).
8. Wang, Y. et al. Quantification of increased cellularity during inflammatory demyelination. Brain 134, 3587–3598 (2011).
9. Wang, X. et al. Diffusion basis spectrum imaging detects and distinguishes coexisting subclinical inflammation, demyelination and axonal injury in experimental autoimmune encephalomyelitis mice. NMR Biomed. 27, 843–852 (2014).
10. Chiang, C.-W. et al. Quantifying white matter tract diffusion parameters in the presence of increased extra-fiber cellularity and vasogenic edema. NeuroImage 101, 310–319 (2014).
11. Varma, G., Duhamel, G., de Bazelaire, C. & Alsop, D. C. Magnetization Transfer from Inhomogeneously Broadened Lines: A Potential Marker for Myelin. Magn. Reson. Med. 73, 614–622 (2015).
12. Girard, O. M. et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn. Reson. Med. 73, 2111–2121 (2015).
13. Varma, G., Girard, O. M., Prevost, V., Duhamel, G. & Alsop, D. C. Extracting a robust inhomogeneous magnetization transfer (ihMT) rate parameter, ihMT-Rex. in Proc. Int. Soc. Mag. Res. Med vol. 23 (2015).
14. Prevost, V. H. et al. Optimization of inhomogeneous magnetization transfer (ihMT) MRI contrast for preclinical studies using dipolar relaxation time (T1D) filtering. NMR Biomed. 30, e3706 (2017).
15. Varma, G. et al. In vivo measurement of a new source of contrast, the dipolar relaxation time, T1D, using a modified inhomogeneous magnetization transfer (ihMT) sequence. Magn. Reson. Med. 78, 1362–1372 (2017).
16. Cheng, J., Shen, D., Yap, P.-T. & Basser, P. J. Single- and Multiple-Shell Uniform Sampling Schemes for Diffusion MRI Using Spherical Codes. IEEE Trans. Med. Imaging 37, 185–199 (2018).
17. Coupe, P. et al. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans. Med. Imaging 27, 425–441 (2008).
18. Andersson, J. L. R., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 20, 870–888 (2003).
19. Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078 (2016).
20. Myronenko, A. & Song, X. Point-Set Registration: Coherent Point Drift. IEEE Trans. Pattern Anal. Mach. Intell. 32, 2262–2275 (2010).
21. Sengul, G., Watson, C., Tanaka, I. & Paxinos, G. Atlas of the spinal cord: Mouse, rat, rhesus, marmoset and human. (Elsevier, 2012).
Figures