1178
Sodium concentration alterations in spinal cord injury and associations to motor and sensory function1NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, UCL, London, United Kingdom, 2National Hospital For Neurology and Neurosurgery, Queen Square, London, United Kingdom, 3Translational Imaging Group, Centre for Medical Image Computing, Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 4Department of Uro-neurology, National Hospital for Neurology and Neurosurgery, London, United Kingdom
Synopsis
Sodium retention as a consequence of spinal cord injury is thought to impair the regenerative ability of neurons but also reduce damage. Studies have shown that sodium-blockers can lead to improved outcomes in some SCI patients. Here alterations in spinal cord total sodium concentrations in spinal cord injury patients and healthy controls were investigated using sodium MRS. The association of sodium concentration to cross sectional area and ASIA score was also explored.
Introduction
Spinal cord injury (SCI) is known to affect tissue microstructure as well as inducing a physiological response resulting in sodium retention. This retention can affect the function of sodium-potassium channels, impairing the regenerative ability but also reducing damage. Studies have shown that sodium-blockers can lead to improved outcomes in some SCI patients1. The ability to measure changes in sodium ion concentration in vivo may help in determining the pathophysiology of SCI as well as indicating which patients could benefit from the use of sodium-blockers, and assist in testing drug efficacy.Aims
To explore whether sodium magnetic resonance spectroscopy (23Na-MRS) of the cervical spinal cord2 is able to a) detect sodium alterations distal to the site of injury b) determine if any correlation of sodium concentration and cross sectional area (CSA) exist and c) establish whether motor and sensory function scores in patients are associated with sodium concentrations.
Methods
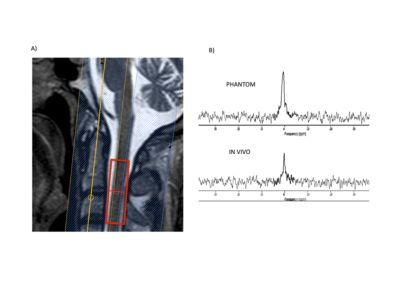
9 healthy controls (6 male, 48±10 years) and 5 SCI patients (4 male, 60±21 years) were recruited. Of these patients one was a spinal cord trauma patient with damage at C4/5 and below. Three were cervical myelopathy (CM) patients awaiting Anterior Cervical Discectomy and Fusion (ACDF) surgery for compression at levels C2-C7. The final subject was post-operative and had already undergone ACDF surgery for cervical myelopathy 2 months prior to scanning (Figure 1). All participants gave informed written consent.23Na-MRS: MRI scans were acquired on a 3T Achieva TX system (Philips Healthcare, Best). Sodium data were acquired using a fixed tuned transmit-receive sodium coil (Rapid, Germany). Using ISIS (Image-Selected In Vivo Spectroscopy), a voxel (9x12x35mm3) centered on the C2-3 intervertebral disc was planned to measure sodium concentration, with TR=300ms, effective TE=0.26ms, 800 averages (Figure 2). Saturation pulses were placed to extend over the edges of the voxel to suppress sodium signal from CSF and bone2. By keeping the voxel distal to the area of spinal cord compression or injury allowed us plan a large enough 23Na-MRS voxel without contamination or artifacts. After scanning each subject, an identical 23Na-MRS scan was run on a reference phantom of similar loading containing 44.8mM sodium for quantification.
Sodium quantification: Data were processed using jMRUI (Figure 2). Signal amplitudes for volunteers and phantom were measured using the AMARES algorithm. Differences in the performance of the ISIS sequence due to differing T1s’ and T2s’ between phantom and tissue were also accounted for, using values from healthy brain tissue. The ratio of the two corrected signals was then used to quantify the sodium concentration for each volunteer2,3,4.
Proton MRI: Proton (1H) gradient echo images, covering the same area as the MRS voxel, were acquired using a 16-channel neurovascular coil for measurement of CSA. Images were acquired in the axial plane containing 10 contiguous slices, FOV=240x180mm2, TR=23ms, TE=5ms, flip angle α=7°, n=8 and 0.5x0.5x5mm3 resolution.
Measurement of CSA: CSA was computed automatically using a SC segmentation algorithm5, which uses an external database of spinal cord images, and their associated segmentations, to fit the cord, providing a mask for each slice. These masks are then used to find the average CSA of the slices covering the 23Na-MRS voxel.
Clinical Assessments: ASIA scores were measured for each patient and broken down into motor and sensory examinations6. For each patient we determined an Upper and Lower Extremity Motor score (UEMS and LEMS) and sensitivity to light-touch or pin-prick.
Results
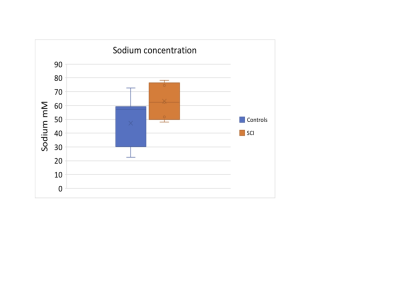
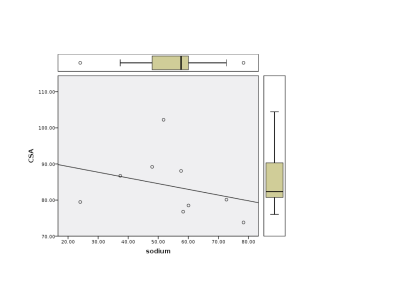
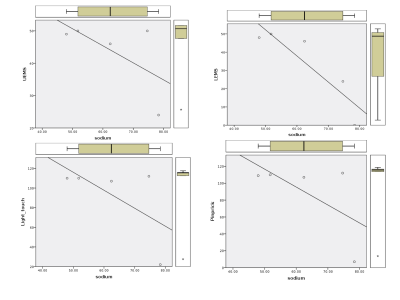
Linear regression showed no significant difference between the HC and SCI group (p=0.074) (Figure 3). However, considering the CM subgroup alone we found a significant increase in sodium concentration (Table 1 p=0.048).No correlations were found between CSA and sodium concentration (p=0.18) (Figure 4), nor with sensory function tests (light-touch (p=0.26), pinprick (p=0.26)) (Figure 5). Whilst UEMS showed no correlation to sodium concentrations (p=0.25), a significant correlation was seen with LEMS (p=0.04).
Discussion
This pilot data shows that sodium can be measured successfully in patients with SCI distal to the site of injury. The lack of correlation between sodium concentration and CSA shows it is unlikely that differences in sodium reported here are due to partial volume effects. The largest increase in sodium concentration was detected in the spinal cord trauma patient, with almost double the sodium concentration compared to HC’s. Elevated sodium concentration was also detected in CM patients awaiting surgery (p=0.048). Sodium concentration in the SCI group correlated to poorer LEMS, showing that even sodium measured distal to the injury site can help predict clinically meaningful scores. Interestingly, the only post-operative subject showed sodium values in the normal range; although this cannot be attributed directly to the surgical intervention, it is encouraging and warrants the need of future pre and post-operative studies.Conclusion
This pilot data is extremely important as it shows that patients with mild or severe spinal cord injury can successfully undergo in vivo sodium quantification distal to the injury site. It shows total sodium concentration is elevated in CM compared to HC. Measurements of sodium concentration may provide further insights into the mechanisms of damage and repair and could also be used to test the efficacy of sodium-channel blockers in improving clinical outcome of interventions.Acknowledgements
Acknowledgments to the UCL-UCLH Biomedical Research Centre for ongoing funding; the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 634541, Spinal Research UK, Wings for Life (Austria), Craig H. Neilsen Foundation (USA) (jointly funding the INSPIRED study), Wings for Life (#169111), the UK Multiple Sclerosis Society (grants 892/08 and 77/2017), and Guarantors of Brain.References
1. Fehlings, M.G., et al., Riluzole for the treatment of acute traumatic spinal cord injury: rationale for and design of the NACTN Phase I clinical trial. J Neurosurg Spine, 2012. 17(1 Suppl): p. 151-62.
2. Solanky BS, Riemer F, Golay X, Wheeler-Kingshott CA, Sodium quantification in the spinal cord at 3T, Magnetic Resonance in Medicine, May 20133.
3. Solanky BS, Riemer F, Golay X, Wheeler-Kingshott, C A.M., FUSS- Fast Ultrashort T2 Sensitive Sodium MRS of the Spinal Cord, Proceedings 21st ISMRM, program number 3514.
4. Riemer F, Solanky BS, Clemence M, Wheeler-Kingshott CAM, Golay X, Bi-Exponential 23Na T2* Components Analysis in the Human Brain ,Proc. 20th ISMRM, 2012, 23225.
5. Prados F, Cardoso MJ, Yiannakas MC, Hoy LR, Tebaldi E, Kearney H, Liechti MD, Miller DH, Ciccarelli O, Wheeler-Kingshot CAM, Ourselin S, Fully automated grey and white matter spinal cord segmentation, Scientific Reports, 20166.
6. Association American Spinal Injury. Standards for Neurological Classification of Spinal Injury Patients.Chicago, IL: American Spinal Injury Association; 1982.
Figures