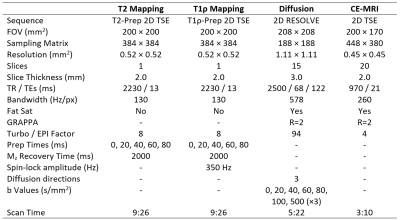
1145
Quantitative T2, T1ρ, and Diffusion Mapping of Early-Stage Ischemic Osteonecrosis of the Femoral Head: An In Vivo Piglet Model Study at 3T MRI1Veterinary Clinical Sciences Department, University of Minnesota, Saint Paul, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Texas Scottish Rite Hospital for Children, Dallas, TX, United States, 4Department of Orthopaedic Surgery, UT Southwestern Medical Center, Dallas, TX, United States, 5Department of Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
This study tested whether T2 and T1ρ relaxation times are sensitive in detecting early-stage osteonecrosis of the femoral head in piglet model under in vivo conditions and at clinical 3T MRI. This study builds on recent ex vivo 9.4T studies assessing T2 and T1ρ in the piglet model. We also evaluated apparent diffusion coefficient for comparison. We found that T2, T1ρ, and ADC were all significantly increased in the ischemic vs. contralateral control femoral heads in n=6 piglets one week after onset of ischemia. These methods may be clinically useful to detect and characterize early-stage osteonecrosis to inform treatment decisions.
Introduction
Osteonecrosis of the femoral head (ONFH) is serious disorder that can cause hip deformity, collapse, and osteoarthritis [1,2]. ONFH accounts for 10% of hip replacements in the United States, typically in young patients (20-40 years old). A major cause of ONFH is interruption of blood supply to the femoral head, as may occur with trauma, Legg-Calvé-Perthes disease, or as a complication of hip surgery. Clinically, there is a need for new imaging techniques that can detect and quantify the extent and severity of ischemic injury to the femoral head at an earlier stage than radiographs and traditional T1- and T2-weighted MRI to enable earlier and more informed treatment decisions. While contrast-enhanced MRI (CE-MRI) can detect a lack of femoral head perfusion [3,4], it provides limited information regarding the extent of bone and marrow injury. Furthermore, growing concerns about the safety of gadolinium contrast agents are increasing the need for alternative, non-contrast-enhanced methods. It was recently demonstrated in ex vivo 9.4T MRI studies of a piglet model of ischemic ONFH that quantitative mapping of T2 and T1ρ relaxation times are sensitive in detecting injury to the femoral head as early as 48 hours after onset of ischemia [5,6]. However, these studies were conducted ex vivo and at ultrahigh-field strength. The purpose of this study was to assess the sensitivity of T2 and T1ρ relaxation times in detecting ischemic injury to the femoral head in the piglet model under in vivo conditions at clinical 3T MRI. We hypothesized that T2 and T1ρ relaxation times would increase due to bone and marrow injury one week following onset of ischemia. We also acquired CE-MRI (to confirm whole femoral head ischemia) and diffusion-weighted MRI (an alternative non-contrast-enhanced approach to detect ischemic injury to the femoral head) [7-9].Methods
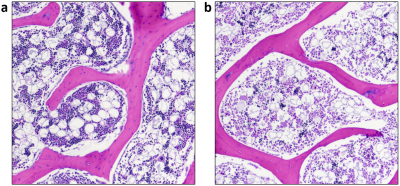
Animals and 3T MRI: Our study was approved by our institutional animal care and use committee. N=6 six-week-old piglets (3 male, 3 female) underwent surgery to induce unilateral whole femoral head ischemia by placing a tight ligature around the femoral neck and transecting the ligamentum teres [10]. The contralateral femoral head was unaltered and served as a control. One week after surgery, the piglets were imaged in vivo at 3T MRI (Siemens Prisma) under general anesthesia. Both hips were imaged simultaneously with: (i) T2 and T1ρ mapping using a single-slice 2D TSE sequence for high SNR and resolution; (ii) diffusion-weighted imaging at multiple b-values using a 2D multi-slice RESOLVE sequence; and (iii) subtraction CE-MRI with a 2D TSE sequence, acquired before and 2.5 minutes after intravenous administration of 0.2 mmol/kg ProHance. Imaging parameters are shown in Table 1. The central 2D imaging slice was positioned such that it passed through the center of both femoral heads.Histology: Five of the piglets were immediately euthanized following imaging, and the femoral heads were harvested for histological analysis. The sixth pig was recovered for subsequent in vivo study. The harvested femoral heads were bisected, fixed in 10% NBF, decalcified in 10% EDTA, cut into 3mm-thick sections, and routinely processed into paraffin blocks for H&E staining. Histological assessment was performed by a board-certified veterinary pathologist.
Data Analysis: 2D T2, T1ρ, and ADC maps at the same central 2D slice location were generated by fitting the echo times, spin-lock times, and b-values (respectively) to a mono-exponential decay model using Matlab. A single 2D region of interest (ROI) was defined for the epiphyseal bone and marrow. Median T2, T1ρ, and ADC values were compared for the ischemic vs. control femoral heads using paired t-tests (p<0.017 considered statistically significant after Bonferroni correction for three comparisons).
Results
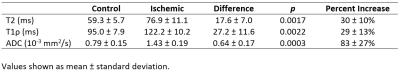
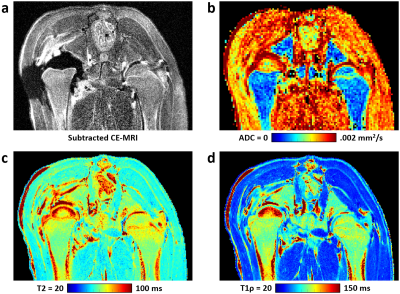
Whole femoral head ischemia was successfully induced in all piglets, as confirmed by lack of gadolinium contrast enhancement in the operated femoral head. T2, T1ρ, and ADC values were increased in the ischemic vs. control femoral head for all six piglets. CE-MRI and quantitative images for one of the piglets are shown in Figure 1. Results of the ROI analysis are shown in Table 2. Both T2 and T1ρ relaxation times were significantly increased in the ischemic femoral heads (p=0.0017 and 0.0022, respectively). The percent increases in the T2 and T1ρ relaxation times were similar (30 ± 10% and 29 ± 13%, respectively). ADC was also significantly increased in the ischemic femoral heads (p=0.0003), on average by 83 ± 27%. Histologically, the ischemic femoral heads had extensive bone marrow necrosis accompanied by early necrosis of osteocytes (Figure 3).Discussion
Our findings support that T2 and T1ρ mapping are sensitive in detecting ischemic injury to bone and marrow in vivo and at clinical 3T field strength. Furthermore, our findings corroborate prior reports that ADC mapping is another sensitive approach to assess early-stage ischemic injury to the femoral head [7-9]. Further study is needed to determine the underlying biological mechanisms driving the sensitivity of each of these methods, as they may potentially provide complementary information.Conclusion
T2, T1ρ, and ADC mapping are sensitive methods to detect early-stage ischemic ONFH. These methods may be clinically useful for earlier detection and quantification of bone and marrow injury to inform treatment decisions.Acknowledgements
We thank Dee Koski, Kathy Stuebner, Amber Winter, Kelly Bergsrud, Andrea Chehadeh, and Sara Pracht for their assistance with the animal studies. This study was supported by the National Institutes of Health (K01AR070894, K01OD021293, and P41EB027061). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
1. Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop 2015; 6(8):590-601.
2. Larson E, Jones LC, Goodman SB, Koo KH, Cui Q. Early-stage osteonecrosis of the femoral head: where are we and where are we going in year 2018? Int Orthop 2018; 42(7):1723-1728.
3. Sebag G, Ducou Le Pointe H, Klein I, Maiza D, Mazda K, Bensahel H, Hassan M. Dynamic gadolinium-enhanced subtraction MR imaging--a simple technique for the early diagnosis of Legg-Calvé-Perthes disease: preliminary results. Pediatr Radiol 1997; 27(3):216-20.
4. Kim HK, Wiesman KD, Kulkarni V, Burgess J, Chen E, Brabham C, Ikram H, Du J, Lu A, Kulkarni AV, Dempsey M, Herring JA. Perfusion MRI in early stage of Legg-Calvé-Perthes disease to predict lateral pillar involvement: a preliminary study. J Bone Joint Surg Am 2014; 96(14):1152-1160.
5. Johnson CP, Wang L, Tóth F, Aruwajoye O, Carlson CS, Kim HK, Ellermann JM. Quantitative MRI helps to detect hip ischemia: preclinical model of Legg-Calvé-Perthes disease. Radiology 2018; 289(2):386-395.
6. Johnson CP, Toth F, Carlson CS, Zbyn S, Ludwig KD, Kim HK, Ellermann JM. T1ρ and T2 relaxation times are sensitive to ischemic injury in femoral head specimens from a piglet model of avascular necrosis independent of a freeze/thaw cycle. Proc 27th ISMRM 2019; No. 1428.
7. Menezes NM, Connolly SA, Shapiro F, Olear EA, Jimenez RM, Zurakowski D, Jaramillo D. Early ischemia in growing piglet skeleton: MR diffusion and perfusion imaging. Radiology 2007; 242(1):129-136.
8. Yoo WJ, Kim YJ, Menezes NM, Cheon JE, Jaramillo D. Diffusion-weighted MRI reveals epiphyseal and metaphyseal abnormalities in Legg-Calvé-Perthes disease: a pilot study. Clin Orthop Relat Res 2011; 469(10):2881-2888.
9. Ozel BD, Ozel D, Ozkan F, Halefoglu AM. Diffusion-weighted magnetic resonance imaging of femoral head osteonecrosis in two groups of patients: Legg-Perthes-Calve and Avascular necrosis. Radiol Med 2016; 121(3):206-13.
10. Kim HK, Su PH. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg Am 2002; 84-A(8):1329-34.
Figures