1142
Knee Epiphyseal Bone Marrow Perfusion Imaging Using FAIR RESOLVE1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Perfusion imaging can provide critical information to assess vasculature status and tissue viability of knee bone marrow. Arterial spin labeling, as a non-invasive and non-contrast-enhanced perfusion imaging approach, is well-suited for routine assessment of bone marrow perfusion, longitudinal monitoring of disease progression and repeated evaluation of therapy response. Recently, knee epiphyseal bone marrow ASL imaging has been demonstrated at 3T with promising results by using FAIR ss-FSE method. However, the ss-FSE image readout only supports single-slice acquisitions. To overcome this limitation, we implemented and evaluated FAIR RESOLVE for multi-slice knee epiphyseal bone marrow perfusion imaging.
Purpose
Bone marrow vasculature and perfusion have a significant role in bone development and repair, and in the initiation, progression and treatment of skeletal diseases (1-3). Frequently, traumatic injuries to the knee lead to significant bone diseases (4) and often concomitant bone marrow lesions occur (5) that may include vascular pathologies. Perfusion imaging can provide critical information to assess vasculature status and tissue viability of knee bone marrow. Arterial spin labeling (ASL) (6), as a non-invasive and non-contrast-enhanced perfusion imaging approach, is well-suited for routine assessment of bone marrow perfusion, longitudinal monitoring of disease progression and repeated evaluation of therapy response. However, ASL imaging of knee bone marrow is challenging since ASL imaging is a low signal-to-noise ratio (SNR) technique and knee bone marrow perfusion is lower than that of the brain or kidneys (7). In adults, the bone marrow in the knee is primarily yellow marrow (8,9), which has a sparse network of capillaries in contrast to red marrow that has an abundant and arborized vascular network (10,11). Recently, knee epiphyseal bone marrow ASL imaging has been performed at 3T with promising results by using flow-sensitive alternating inversion recovery (FAIR) (12) for labeling and a single-shot fast spin echo (ss-FSE) sequence for image readout (referred to as FAIR ss-FSE) (13). However, the ss-FSE image readout only supports single-slice acquisitions. To overcome this limitation, we implemented and evaluated a multi-slice ASL imaging sequence for knee epiphyseal bone marrow perfusion imaging.Methods
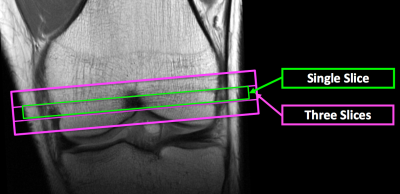
The implemented multi-slice knee ASL imaging sequence used the same ASL method as that of the FAIR ss-FSE (13) except that readout-segmented EPI (RESOLVE) (14) was used as image readout to take advantage of its insensitivity to susceptibility-associated artifacts and distortion (referred to as FAIR RESOLVE). Studies with healthy adults were performed on a Siemens 3TMRI scanner using a single-channel transmit and 15-channel receive knee coil under an IRB approved protocol with informed written consent. Perfusion imaging studies used both FAIR ss-FSE and FAIR RESOLVE with a single slice, and FAIR RESOLVE with three slices. The imaging slice positions are illustrated in Figure 1. To ensure at least ¾ coverage of the knee coil over the labeling region, the knees of the participants were positioned with the proximal margin of superior pole of the patella positioned at the center of the coil.Major parameters specific for the ss-FSE image readout were: TE=21 ms; phase-encoding direction=left-to-right; partial Fourier=4/8; and total acquisition time=~5 mins. Major parameters specific for the RESOLVE image readout were: TE1/TE2=44/76 ms; phase-encoding direction=anterior-posterior; partial Fourier=5/8; number of segments=3; and total acquisition time=~10 mins. Common parameters for single-slice imaging using two methods were: TR=3000 ms; resolution=2x2x10 mm3; slab thickness for labeling/control inversion=210/30 mm; labeling time/total delay time=600/1200 ms; slab thickness/RF duration/interval of proximal post-bolus saturations=40 mm/25 ms/50 ms; pairs of label and control images=50; and number of M0 images acquired at the end of ASL series=2. For both imaging methods, image acquisition employed fat saturation and the signal gain was increased to boost the water signal level in the bone marrow. For multi-slice FAIR RESOLVE imaging, the same parameters as those for single-slice acquisitions were used except the following: slab thickness for labeling/control inversion=210/50 mm; and imaging slice thickness=8 mm with 25% gap. For each ASL scan, 200 noise images were acquired after the perfusion acquisition by turning off RF pulses to facilitate perfusion SNR analysis (15).
Results and Discussion
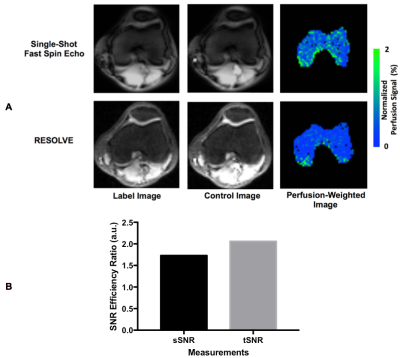
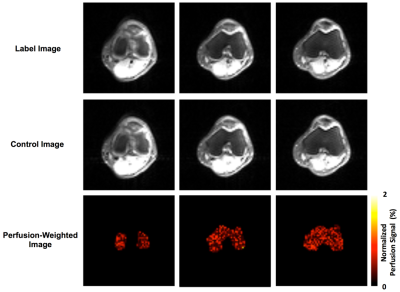
Perfusion imaging results from one subject using both the FAIR ss-FSE and FAIR RESOLVE methods are presented in Figure 2. The ss-FSE image readout provided higher perfusion image quality than RESOLVE with higher spatial and temporal SNR efficiencies. This indicates that whenever single-slice coverage is sufficient for a study, ss-FSE is still the preferred image readout. Figure 3 shows perfusion imaging results from one subject using the multi-slice FAIR RESOLVE method. These results suggest that multi-slice knee epiphyseal bone marrow perfusion imaging is feasible, but the long acquisition time makes it challenging to apply such a method for clinical applications.We also evaluated other alternative imaging readouts for multi-slice knee ASL imaging, such as echo planar imaging (EPI) and spin-echo echo planar imaging (SE-EPI). Compared to RESOLVE, both imaging methods were quite sensitive to B0 inhomogeneity and susceptibility. B0 inhomogeneity and susceptibility are expected to be significantly larger in the knee due to its trabecular microarchitecture and bone / fat interfaces than those in the brain and kidneys. Knee ASL imaging using EPI and SE-EPI readouts resulted in significant, unacceptable susceptibility-associated ghosting artifacts and distortion.
Conclusions
We have demonstrated the feasibility of multi-slice knee epiphysealbone marrow ASL imaging using FAIR RESOLVE. However, there are major limitations at 3T. To acquire high-quality ASL perfusion data for FAIR RESOLVE, respective tradeoffs were lower resolution, a large number of averages and a long total acquisition time. To overcome these limitations, performing such studies at 7T is a promising next step to boost imaging SNR and take advantage of the prolonged blood and tissue relaxation times to benefit ASL imaging and reduce total imaging time.Acknowledgements
P41 EB027061, R01AR070020, K01 AR070894, and UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
1. Filipowska J, Tomaszewski KA, Niedzwiedzki L, Walocha JA, Niedzwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017;20(3):291-302.
2. Marenzana M, Arnett TR. The Key Role of the Blood Supply to Bone. Bone Res 2013;1(3):203-215.
3. Stegen S, van Gastel N, Carmeliet G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone 2015;70:19-27.
4. Selvarajah L, Curtis AM, Kennedy OD. Bone Microdamage in Acute Knee Injury. Curr Rheumatol Rep 2018;20(12):89.
5. Kon E, Ronga M, Filardo G, Farr J, Madry H, Milano G, Andriolo L, Shabshin N. Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg Sports Traumatol Arthrosc 2016;24(6):1797-1814.
6. Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 1992;23(1):37-45.
7. McCarthy I. The physiology of bone blood flow: a review. J Bone Joint Surg Am 2006;88 Suppl 3:4-9.
8. Cristy M. Active bone marrow distribution as a function of age in humans. Phys Med Biol 1981;26(3):389-400.
9. Piney A. The anatomy of the bone marrow with special reference to the distribution of the red marrow. Br Med J 28 (1922): 792-795.
10. Travlos GS. Normal structure, function, and histology of the bone marrow. Toxicol Pathol 2006;34(5):548-565.
11. Custer RP (1932) Studies on the structure and function of bone marrow. J Lab Clin Med 17: 951-962.
12. Kim SG, Tsekos NV. Perfusion imaging by a flow-sensitive alternating inversion recovery (FAIR) technique: application to functional brain imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 1997;37(3):425-435.
13. Li X, Johnson CP, and Ellermann J. Epiphyseal Bone Marrow Perfusion Imaging in the Distal Femur Using Arterial Spin Labeling: Feasibility and Challenges. Proc. Intl. Soc. Mag. Reson. Med. 25 (2017): 0852.
14. Holdsworth SJ, Skare S, Newbould RD, Guzmann R, Blevins NH, Bammer R. Readout-segmented EPI for rapid high resolution diffusion imaging at 3 T. Eur J Radiol 2008;65(1):36-46.
15. Li X, Wang D, Auerbach EJ, Moeller S, Ugurbil K, Metzger GJ. Theoretical and experimental evaluation of multi-band EPI for high-resolution whole brain pCASL Imaging. NeuroImage 2015;106:170-181.
Figures