1141
Diffusion Tensor Imaging detects recovery after acute muscle injury1Department of Biomedical Engineering & Physics, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands, 2Department of Radiology & Nuclear Medicine, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands, 3Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 4Department of Orthopaedic Surgey, University Medical Center Utrecht, Utrecht, Netherlands, 5Department of Orthopaedic Surgey, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands
Synopsis
41 athletes with an acute hamstring injury underwent MRI examination of their injured leg and the uninjured contralateral leg at three different time points: (1) within one week after the index injury (baseline), (2) two weeks after baseline, and at (3) Return to Play (RTP). Baseline DTI values (MD, RD and the three eigenvalues) were elevated compared to control hamstring muscles and decreased during the RTP phase. qT2 values were elevated after the index injury and did not change over time. DTI is promising for monitoring recovery of hamstring injuries.
Introdcution
Hamstring injuries frequently occur in recreational and elite sports, contribute to of 37% all muscle injuries1,2. Currently, T2-weighted MRI is used to diagnose, grade and classify muscle injury. It is however impossible to estimate the time needed for return to play (RTP) from these T2-weighed scans, which is essential for preventing re-injury or unnecessary long rehabilitation periods3,4. Previous research has shown that quantitative T2 (qT2) and Diffusion Tensor Imaging (DTI) may be able to asses acute muscle injury recovery5. The aim of this study was to evaluate the potential of DTI and qT2 values as outcome measures for monitoring recovery after an acute hamstring injury from injury until return to play.Methods
MRI datasets were acquired in the thigh muscles of 66 athletes using a 3T MR scanner (Ingenia, Philips, Best, The Netherlands) at three different time points: (1) within one week after sustaining the injury, (2) two weeks after baseline, and at (3) at Return to Play (RTP) where RTP is defined as full training at pre-injury level. The MR examination consisted of diffusion weighted SE-EPI for DTI parameter estimation, an MSE sequence for quantitative T2 mapping and a FS T2-weighted sequence for muscle injury grading (See Table 1 for detailed sequence parameters). All datasets were processed using QMRITools for Wolfram Mathematica (https://github.com/mfroeling/QMRITools)6. Diffusion data were denoised and registered to correct for motion and eddy currents. The diffusion tensor was calculated using an Intra Voxel Incoherent Motion (IVIM) based iterative Weighted Linear Least Squares algorithm (iWLLS)7,8. qT2 maps were calculated using an extended phase graph (EPG) fit, considering different relaxation times for the water and fat signal components9. Manual segmentation of the injured hamstrings muscles and contralateral uninjured control muscle was performed in ITK-snap. ROIs consisted of 7 slices (35mm) overlaying the origin of the injury. The DTI parameters (mean diffusivity (MD), radial diffusivity (RD), λ1, λ2, λ3 and fractional anisotropy (FA)) and qT2 were calculated for each subject at each time point. Time course changes over the three time points were assessed with a multi-level linear mixed model. Statistical significance level was corrected for multiple comparisons and therefore set to p<0.007. Post-hoc analysis was used to determine the contributions of the different time points. Paired t-tests were used to detect differences between injured and uninjured muscles at every time point.Results
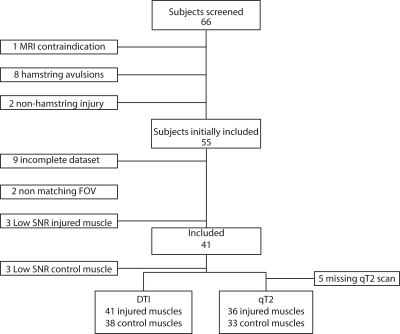
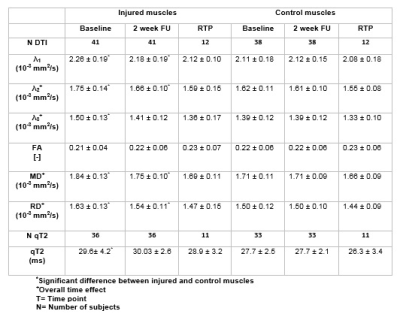
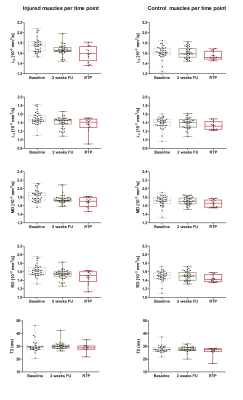
Out of the 66 athletes, 25 athletes were excluded due to full thickness free tendon injuries or lack of a MRI positive scan. 41 athletes (age 27.8±7 years; 2 females/39 males) with an acute hamstring injury grade 0-2 (35 Biceps Femoris Long head muscles, five Semimembranosus muscles and 1 Semitendinosus muscle) were included in this study. The mean time to RTP was 50 days. Multi-parametric images and quantitative parameter maps of three representative athletes are shown in Figure 1. Out of 41 athletes analyzed, 39 athletes were included for the qT2 analysis and 41 for the DTI analysis (Figure 2 for flow chart). Mean values and standard deviations of all outcome parameters for the three time points per muscle are summarized in Table 2 and corresponding boxplots are plotted in Figure 3. At baseline, all DTI parameters (p<0.001) and qT2 values (p=0.04) were significantly elevated compared to control muscles. Two weeks after baseline, MD (p=0.002), RD (p=0.02), λ1 (p=0.007) and λ2 (p=0.003) were significantly higher for the injured muscles. FA (p=0.62), λ3 (p=0.21) and qT2 (p=0.09) showed no differences compared to the control muscles. At RTP (n=24), none of the DTI or qT2 values showed a difference between the control and injured muscles. Significant time course changes were found for MD (p<0.001), RD (p<0.001), λ2 (p<0.001) and λ3 (p=0.001) in injured muscles but not in the uninjured contralateral leg. FA (p=0.40), λ1 (p=0.02), and qT2 (p=0.61) showed no significant time effect for the injured nor the control muscles.Discussion
Both DTI parameters and qT2 values were significantly elevated following acute hamstring injury. DTI parameters normalized during the return to play recovery period, whereas qT2 values remained elevated at RTP. The elevated DTI values found directly after acute muscle injury agree with previous work in calf and Hamstring injuries and have been associated with more free water and modifications in muscle micro-architecture10-12. Combined, these findings indicate that DTI can potentially be used to monitor muscle injury recovery and might guide the return to play decision process. However, important to note is that, although our results are promising on group level basis, for now it is unclear what the value of this technique will be for the individual athlete. Additionally, it is yet to be determined what the additional predictive diagnostic value of DTI is compared to clinical measures. Future work should focus on establishing quantitative correlations between these MRI parameters and clinical measures such as RTP time and re-injury rates. Lastly, correcting mean values qT2 and DTI for the severity and location (myotendinous junction, tendon, myofascial) of the injury could also give us more insights in the recovery process.Conclusion
Our DTI measures proved sensitive to detect time-course changes during the recovery process of acute hamstring injuries.Acknowledgements
No acknowledgement found.References
1. Brooks JHM: Incidence, Risk, and Prevention of Hamstring Muscle Injuries in Professional Rugby Union. Am J Sports Med 2006; 34:1297–1306.
2. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professionnal football (soccer). Am J Sports Med. American Orthopaedic Society for Sports Medicine; 2011; 39 (6) 1226-1232
3. Reurink G, Whiteley R, Tol JL: Hamstring injuries and predicting return to play: “bye-bye MRI?”. Br J Sports Med 2015; 49:1162–3.
4. Wangensteen A, Almusa E, Boukarroum S, et al.: MRI does not add value over and above patient history and clinical examination in predicting time to return to sport after acute hamstring injuries: a prospective cohort of 180 male athletes. Br J Sports Med 2015; 49:1579–87.
5. Froeling M, Oudeman J, Strijkers GJ, et al.: Muscle Changes Detected by Diffusion-Tensor Imaging after Long-Distance Running. Radiology 2014; 274:140702.
6. Froeling M: QMRTools: a Mathematica toolbox for quantitative MRI analysis. J Open Source Softw 2019; 4(38),1204.
7. Veraart J, Sijbers J, Sunaert S et al. Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. Neuroimage 2013; 81:335–346. doi: 10.1016/j.neuroimage.2013.05.028.
8. Schlaffke L, Rehmann R, Rohm M, et al.: Multi‐center evaluation of stability and reproducibility of quantitative MRI measures in healthy calf muscles. NMR Biomed 2019; 32:e4119.
9. Marty B, Baudin P-Y, Reyngoudt H, et al.: Simultaneous muscle water T2 and fat fraction mapping using transverse relaxometry with stimulated echo compensation. NMR Biomed 2016; 29:431–43.
10. Giraudo C, Motyka S, Weber M, et al.: Normalized STEAM-based diffusion tensor imaging provides a robust assessment of muscle tears in football players: preliminary results of a new approach to evaluate muscle injuries. Eur Radiol. 2018 Jul;28(7):2882-2889.
11. Zaraiskaya T, Kumbhare D, Noseworthy MD. Diffusion tensor imaging in evaluation of human skeletal muscle injury. J Magn Reson Imaging. 2006;24(2):402 -408
12. Fulford J, Eston RG, Rowlands A V., Davies RC. Assessment of magnetic resonance techniques to measure muscle damage 24h after eccentric exercise. Scand J Med Sci Sport; 2015;25(1)
Figures