1129
Temperature Triggered Release of a Protein from Thermoresponsive Nanogels Using Thermal Magnetic Resonance1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), Berlin, Germany, 2Physikalisch-Technische Bundesanstalt (PTB), Berlin, Germany, 3Institute of Chemistry and Biochemistry, Freie Universität Berlin, Berlin, Germany, 4Instituto de Desarrollo Tecnológico para la Industria Química (INTEC), Universidad Nacional del Litoral (UNL) - Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Santa Fe, Argentina, 5POLYMAT and Applied Chemistry Department, Faculty of Chemistry, University of the Basque Country UPV/EHU, Donostia-San Sebastián, Spain, 6IKERBASQUE, Basque Foundation for Science, Bilbao, Spain, 7Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany
Synopsis
Thermal Magnetic Resonance (ThermalMR) adds a thermal dimension to an MR device by exploiting the constructive interference of radiofrequency (RF) waves for temperature intervention. Here, the capacity of ThermalMR is demonstrated in a model system involving the release of a protein from thermoresponsive nanogels. Upon RF heating the nanogels (T=43°C), 29.3% of the protein were released after 6h which is in accordance with the release profile obtained for the reference data derived from a water bath setup. ThermalMR provides an ideal testbed for the study of temperature induced release of drugs, MR probes and other agents from thermoresponsive carriers.
Introduction
Temperature is a physical parameter with diverse biological implications and increasing clinical relevance. Magnetic Resonance (MR) is a mainstay of diagnostic imaging. Ultrahigh field MR (B0≥7.0T) employs higher radio frequencies (RF) than conventional MR and has unique potential to provide high-resolution imaging, temperature monitoring (MR thermometry, MRTh) and controlled temperature manipulation, denominated as Thermal Magnetic Resonance (ThermalMR)1,2. Recognizing this opportunity, this study examines the feasibility of ThermalMR for controlled release of a protein from thermoresponsive nanogels at 7.0T.Materials and Methods
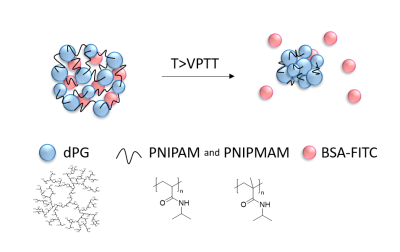
The themoresponsive nanogels (Fig.1), with a volume phase transition temperature (VPTT) of 38°C, were synthesized using precipitation polymerization of acrylated dendritic polyglycerol (Ac-dPG) as a macromolecular cross-linker and temperature-sensitive polymers poly(N-isopropylacrylamide) (PNIPAM) and poly(N-isopropyl methacrylamide) (PNIPMAM) as linear counterpart3,4.Bovine serum albumin labeled with fluorescein (BSA-FITC) was encapsulated in the nanogels by letting dry nanogels (5mg) swell in 1mL of a BSA-FITC solution (0.5mg/mL, pH=7.4) for at least 24h at 4°C. The solution was purified three times by centrifugal filtration5 (MWCO=300kDa, 10min, 4800xg).
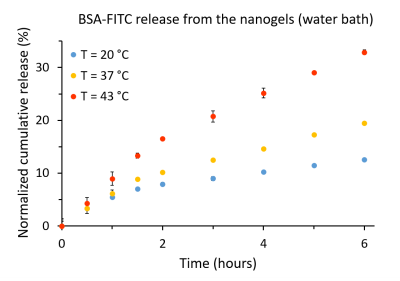
The release profile of the BSA-FITC was firstly assessed using a water bath as a heat source. The above-mentioned nanogel BSA-FITC solution was diluted to 1mg/mL of nanogel and then distributed into three centrifugal filters6. The filters were placed in water bath at 20°C (room temperature), 37°C and 43°C. At certain time intervals, the samples were centrifuged (10min, 4800xg) and the filtrates were taken for fluorescence analysis7 (λex/λem=490/525nm).
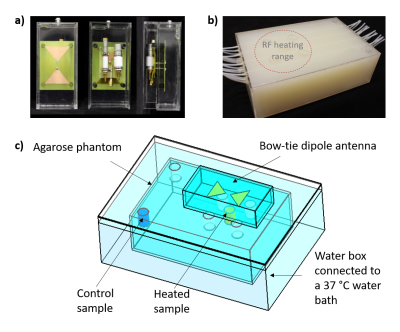
Next, the release of the BSA-FITC was assessed using ThermalMR in a 7.0T whole-body MR scanner8. The experimental setup (Fig.2) included i) a bow-tie dipole RF antenna1 for imaging, temperature monitoring (MRTh) and RF heating, ii) a agarose phantom (σ=1.03S/m, εr=71.9) with seven sample holders: five in the heating area of an RF antenna (heated sample), and two outside the heating range (control sample), iii) a water box connected to a water bath at 37°C, iv) a customized high-power Tx/Rx switch9 and v) fiber optic temperature sensors10 (FOTS). Nanogel BSA-FITC solution (1mg/mL) was placed in three centrifugal filters6; 1) in the heated sample holder, 2) in the control sample holder and 3) outside the MRI scanner room at room temperature (20°C).
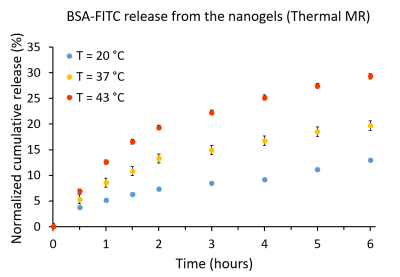
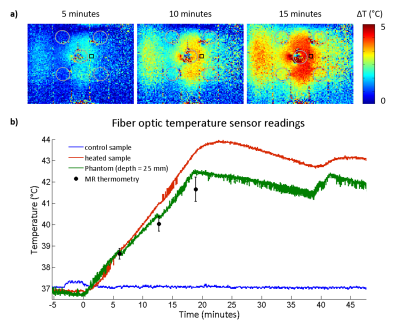
The RF heating paradigm consisted of RF pulses (t=4ms|U=280V|TR=40ms|duty cycle=10%) delivering an average power (Pavg)=100W at the RF antenna’s feeding point for 15min interleaved with 2D MRTh for every 5min. For MRTh, a PRF shift method11 with a dual gradient-echo technique12,13 was employed (FOV=(290×290)mm2|TR=102ms|TE1/TE2=2.26/11.44ms| spatial resolution=(1.5×1.5×4)mm3|nominal flip angle=30°). Three FOTS (placed in the heated sample holder, the control sample holder, and inside the phantom at depth=25mm) were used as an external reference for the MRTh. After reaching a temperature of 43°C in the heated sample holder, the temperature was maintained with subsequent RF pulses for 1-2min. At certain time intervals, centrifugal filters were taken, centrifuged and the filtrates were analysed.
Results
For the water bath heating reference (Fig.3), at 20°C (18°C bellow VPTT), the release of the BSA-FITC from the nanogels was 12.5% after 6h and 14.1% after 19h. At 37 °C (1°C below VPTT, nanogels already started to shrink), the release was 19.5% after 6h and 27.6% after 19h. At 43 °C (5°C above VPTT, nanogels fully shrunken), the release was boosted to 32.8% after 6h and 43.6% after 19h.The BSA-FITC release profile obtained for the ThermalMR setup was found to be in accordance with the release kinetics from the water bath setup (Fig.4): at 20°C, the release was 12.9% after 6h. At 37°C, the release was 19.6% after 6h. At 43°C, the release was raised to 29.3% after 6h.
The temperature changes induced in the phantom due to RF heating were accessed with readings from FOTS and with MR thermometry (MRTh), Fig.5. In the heated sample, the FOTS registered T=38.8°C, T=40.9°C and T=43.4°C after 5min, 10min and 15min of RF heating, respectively. In the control sample, the FOTS registered a constant T=37°C. In the agarose gel phantom, at a depth of 25 mm, a T=38.8 °C (FOTS) and T=38.6 °C (MRTh) was observed after 5min of RF heating. After 10 minutes, it increased to T=40.5°C (FOTS) and T=40.0°C (MRTh). After 15min, temperature readings were T=42.5 °C (FOTS) and T=41.7 °C (MRTh).
Discussion and conclusion
This work demonstrated the feasibility of RF heating induced release of BSA-FITC from thermoresponsive nanogels using an integrated ThermalMR setup for anatomic reference imaging, temperature monitoring, and thermal intervention. The release profile obtained for the reference data derived from a water bath setup used for temperature intervention is in accordance with the release kinetics deduced from the ThermalMR setup. Temperature monitoring with MRTh and fiber optic temperature sensors were well correlated with a deviation of 0.2-0.7°C. These findings support the feasibility of ThermalMR for temperature controlled release of cargo from thermoresponsive nanocarriers. In conclusion, ThermalMR adds a thermal intervention dimension to an MRI device and provides an ideal testbed for the study of temperature-induced release of guest molecules from thermoresponsive carriers. Integrating diagnostic imaging, temperature intervention and temperature response control with ThermalMR is conceptually appealing for the study of the role of temperature in biology and disease and for the pursuit of personalized therapeutic drug delivery approaches for better patient care.Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme under grant agreement No 743077 (ThermalMR), and by the Bundesministerium für Bildung und Forschung (BMBF) through the NanoMatFutur program (13N12561).References
1. Winter, L. et al. Design and evaluation of a hybrid radiofrequency applicator for magnetic resonance imaging and RF induced hyperthermia: electromagnetic field simulations up to 14.0 Tesla and proof-of-concept at 7.0 Tesla. PLoS One 8, e61661 (2013).
2. Winter, L. et al. Thermal magnetic resonance: physics considerations and electromagnetic field simulations up to 23.5 Tesla (1GHz). Radiat. Oncol. 10, 201 (2015).
3. Cuggino, J. C. et al. Thermosensitive nanogels based on dendritic polyglycerol and N-isopropylacrylamide for biomedical applications. Soft Matter 7, 11259–11266 (2011).
4. Theune, L. E. et al. Critical parameters for the controlled synthesis of nanogels suitable for temperature-triggered protein delivery. Mater. Sci. Eng. C 100, 141–151 (2019).
5. Vivaspin 6, Sartorius AG, Göttingen, Germany.
6. Vivaspin 500, Sartorius AG, Göttingen, Germany.
7. Tecan, Infinite 200 Pro, Männedorf, Switzerland.
8. Siemens Healthineers, Erlangen, Germany.
9. Ji, Y. et al. High peak and high average radiofrequency power transmit/receive switch for thermal magnetic resonance. Magn. Reson. Med. 80, 2246–2255 (2018).
10. Omniflex, Neoptix, Quebec, Canada.
11. Ishihara, Y. et al. A precise and fast temperature mapping using water proton chemical shift. Magn. Reson. Med. 34, 814–823 (1995).
12. Rieke, V. & Butts Pauly, K. MR thermometry. J. Magn. Reson. Imaging An Off. J. Int. Soc. Magn. Reson. Med. 27, 376–390 (2008).
13. Wonneberger, U. et al. Intradiscal temperature monitoring using double gradient‐echo pulse sequences at 1.0 T. J. Magn. Reson. Imaging 31, 1499–1503 (2010).
Figures