1120
Impact of respiration on B1+ field and SAR distribution at 7 T using a novel EM simulation setup1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
At 7T body imaging spatial variations of the transmit magnetic (B1+) and electric (E) fields are observed. Additionally, recent in-vivo studies showed that B1+ patterns vary throughout the respiratory cycle. We present a novel electromagnetic (EM) simulation setup that allows investigating respiration-induced changes of the E- and B1+ fields. Using such simulations, we aim to verify the aforementioned in-vivo results that demonstrated respiration-induced changes of B1+ and corresponding flip angle distributions in the heart. Furthermore, the hitherto neglected, corresponding SAR variations are investigated and we find an up to 100 % change in local SAR throughout the respiratory cycle.
Introduction
A fundamental challenge of MRI at ultra-high fields (UHF$$$\,\geq\,$$$7T) results from spatial variations of the magnetic$$$\:(B^+_1)\:$$$and electric (E) transmit radiofrequency (RF) fields leading to spatially varying flip angles (FA) and power deposition as measured by the specific absorption rate (SAR). In addition, recent studies showed that at 3T and 7T the FA pattern and amplitude can change as a function of the respiratory position$$$^{\textrm{1,2,3}}.\:$$$Particularly, homogeneous FA excitations during exhalation can exhibit FA dropouts during inhalation in the same region of interest (ROI)$$$^1.\:$$$This finding implies that the E-field pattern will also vary temporally during respiration suggesting that peak spatial SAR (psSAR) may change as well. This aspect of respiration related effects has not been investigated to date.Thus, the present work pursues three aims. First, we present a novel electromagnetic (EM) simulation setup that allows investigating respiration-induced changes of the E- and$$$\:B^+_1$$$-field. Second, using EM simulations we aim to verify the respiration-induced$$$\:B^+_1\:$$$changes found in-vivo. Finally, the simulations are used to explore the time-varying effects of respiration on psSAR.
Methods
Finite-difference time-domain (FDTD) EM simulations were performed with Sim4Life$$$^5\:$$$using a 7T 16-channel Tx/Rx body coil$$$^8\:$$$(cf. Fig.1) and respiration-resolved body models (inhale, intermediate, exhale). Such models were obtained by XCAT$$$^4,\:$$$a software that allows generating body models at arbitrary respiratory states based on user-defined input parameters (e.g. respiration depth, duration). The RF coil, which consists of four anterior and four posterior blocks each containing a dipole and a loop element, was investigated for two setups: (i) a spatially fixed setup$$$^{6,7,8}$$$, which generates varying minimum distances of 12-30mm between chest wall and coil and (ii) a moving setup$$$^9$$$, i.e. the anterior blocks move with respiration at fixed minimum distance of 10 mm to the body. The positioning was accomplished with best possible accuracy given a 2x2x2 mm3 voxel size.Each dipole consists of a meandering structure and one RF port. The rectangular loops contain five capacitors in series, one RF port and one capacitor parallel to the port, which was implemented by a co-simulation$$$^{10}$$$.
For each of the six simulations (two setups and three respiratory states), complex$$$\:B^+_1$$$ and E-fields were extracted. Loops and dipoles were investigated separately to explore the impact of respiration for each element type. Two phase shim settings were calculated by modifying the phases of channels$$$\:1..k..N.:\:$$$(i) a homogeneous shim$$$^{11}\:$$$that minimizes the$$$\:B^+_1$$$-field's coefficient of variation (CV) \begin{equation}CV=\frac{\text{std}(|\sum_k B^{+}_{1,k}\,\cdot\,\text{exp}(i \varphi_k)|)}{\text{mean}(|\sum_k B^{+}_{1,k}\,\cdot \text{exp}(i \varphi_k)|)},\end{equation}and (ii) an efficient shim that maximizes the transmit efficiency$$$\:\eta\:^{12}$$$\begin{equation}\eta=\frac{|\sum_k B^{+}_{1,k}\,\cdot \text{exp}(i\varphi_k)|}{\sum_k |B^{+}_{1,k}|}.\end{equation}Optimizations were performed within a heart-ROI for exhale in an axial slice through the heart-centre for the fixed coil setup. In addition, a zero-phase shim with$$$\:\varphi_k\,=\,0\:\forall\:k\:$$$was applied. Peak spatial-10g-averaged-SAR$$$\:(\text{psSAR10g})\:$$$was calculated using Sim4Life according to IEEE/IEC62704-1 standard$$$^{13}.\:$$$To illustrate the maximum$$$\:\text{psSAR10g},\:$$$maximum-intensity projections (MIP) along the z-axis are visualized as maps and MIPs along y- and z-axis as line-plots. The analysis was performed with Matlab$$$^{14}\:$$$and Python$$$^{15}\:.$$$
Results
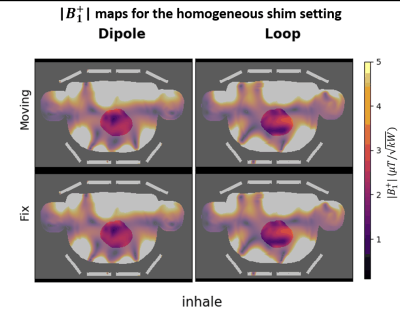
Figs.2,3 demonstrate resulting respiration-induced$$$\:B^+_1\:$$$changes for the three different shims. Line plots along the y-axis, chosen at an x-position (left-right) with large$$$\:B^+_1\:$$$variation, reveals peak$$$\:B^+_1\:$$$changes between exhale and inhale of 50$$$\,\%\:$$$(fixed loop, homogenous shim) and 30$$$\,\%\:$$$(fixed loop, efficiency shim) which agree with findings at 3T using a body coil showing changes of 50$$$\,\%\:$$$ in the human liver$$$^{3}.\:$$$Respiration induced changes increase CV by maximally 38$$$\,\%\:$$$(dipole, homogeneous shim) and reduce$$$\:\eta\:$$$up to$$$\:6\,\%\:$$$(loop, efficient shim) (Tab.1). While$$$\:|B^+_1|\:$$$profiles of the efficiency shims appear rather stretched anterior-posteriorly between inhale and exhale (Fig.2, green arrows), homogeneous shim$$$\:B^+_1\:$$$profiles rather show a local drop in$$$\:B^+_1\:$$$within the heart (37$$$\,\%\:$$$decrease, see green arrow). This local dropout is observed for both, loops and dipoles, as illustrated in animated Fig.3. While similar$$$\:|B^+_1|\:$$$respiration induced changes between exhale and inhale have been observed for the fixed and the moving coil setup within the heart region, differences exist outside the heart, closer to the coil: for the moving coil setup similar$$$\:|B^+_1|\:$$$amplitudes exist for inhale and exhale, whereas for the fixed setup$$$\:|B^+_1|\:$$$decreased by 63$$$\,\%\:$$$from inhale to exhale.Fig.4 shows MIPs of$$$\:\text{psSAR10g}\:$$$of only the anterior body region (shaded area in Fig.4a) along z-direction, and along z and y-direction (Fig4b). The fixed setup shows larger respiration-induced variations of the maximum$$$\:\text{psSAR10g}\:$$$as the moving coil with 96$$$\,\%\:$$$increased$$$\:\text{psSAR10g}\:$$$between inhale/exhale for the loops and 46$$$\,\%\:$$$higher$$$\:\text{psSAR10g}\:$$$for the dipoles (Fig.4b). For the moving setup no substantial changes in$$$\:\text{psSAR10g}\:$$$for inhale were observed, but local increases of the$$$\:\text{psSAR10g}\:$$$pattern of 46$$$\,\%\:$$$(loop, homogeneous shim).
Discussion and Conclusion
This work presents a novel EM simulation setup to investigate respiration-resolved changes of the EM transmit field using respiration-resolved XCAT models. The findings confirm experimental results$$$^{1,2,3}\:$$$showing substantial $$$\:B^+_1$$$ variations between different respiratory states. The work here focussed on$$$\:B^+_1\:$$$shimming, but respiration-induced FA alterations are expected to become even more significant for parallel transmit pulses$$$^1.\:$$$A similar range of$$$\:B^+_1\:$$$variation in the target volume was observed for the moving as for the fixed coil configuration.Presently, most local Tx/Rx coils in use at 7T are in moving configuration and our work demonstrates a favorable$$$\:\text{psSAR10g}\:$$$behavior as a function of the respiratory cycle, compared to the fixed coil setup. For the latter setup we demonstrate that $$$\:\text{psSAR10g}\:$$$-variations between exhale and inhale can increase up to two-fold. The work might inform the design of future 7T body coils as pursued by several groups and is also expected to be relevant for 3T body RF coils.
Acknowledgements
This work was supported by the German Research Foundation (DFG), grant number SCHM 2677/2-1 and by the EMPIR grant 17IND01 MIMAS. The EMPIR initiative is co-funded by the European Union's Horizon 2020 research and innovation programme and the EMPIR Participating States.References
1. Schmitter S et al. Design of Parallel Transmission Radiofrequency Pulses Robust Against Respiration in Cardiac MRI at 7 Tesla, Magn. Reson. Med. 2015; 74:1291–1305
2. Padormo F et al. Assessing and correcting respiration induced variation of B1 in the liver. Proc. Intl. Soc. Mag. Reson. Med. 2009; 17:754
3. Nehrke K et al. Free-breathing abdominal B1 mapping at 3 T using the DREAM approach. Proc. Intl. Soc. Mag. Reson. Med. 2012; 20:3356.
4. W. P. Segars et al., 4D XCAT phantom for multimodality imaging research, Med. Phys. 2010; 37: 4902-4915
5. Sim4Life (5.0.1.4765), ZMT Zurich MedTech AG, Zurich, Switzerland, available at https://zmt.swiss/sim4life/
6. Vaughan J.T. et al, Whole-Body Imaging at 7T: Preliminary Results, Magn. Reson. Med. 2009; 61:244-248
7. Orzada S. et al., A 32-Channel Integrated Body Coil for 7 Tesla Whole-Body Imaging, Proc. Intl. Soc. Mag. Reson. Med. 2016; 24:167
8. Orzada S. et al., 16-channel Tx/Rx body coil for RF shimming with selected Cp modes at 7T, Proc. Intl. Soc. Mag. Reson. Med. 2010; 18:50
9. Ertürk M et al., A 16-Channel Combined Loop-Dipole Transceiver Array for 7 Tesla Body MRI, Magn. Reson. Med. 2017; 77:884–894
10. Kozlov M. et al., Fast MRI coil analysis based on 3-D electromagnetic and RF circuit co-simulation, Journal Magn. Reson. 2009; 200:147-152
11. Schmitter S et al. Cerebral TOF angiography at 7T: Impact of B1+ shimming with a 16-channel transceiver array, Magn. Reson. Med. 2013; 71:966–977
12. Metzger G et al., Local B1+ shimming for prostate imaging with transceiver arrays at 7T based on subject-dependent transmit phase measurements, Magn. Reson. Med. 2008; 59:396–409
13. IEEE/IEC 62704-1 Recommended Practice for Determining the Peak Spatial-Average Specific Absorption Rate (SAR) in the Human Body from Wireless Communications Devices, 30 MHz - 6 GHz: General Requirements for using the Finite Difference Time Domain (FDTD) Method for SAR Calculations, Draft 2011
14. Matlab(R2019b), The Mathworks, Inc., Natick, USA, available at www.mathworks.com
15. Python 3.7, Python Software Foundation, available at http://www.python.org
Figures