1116
Paradoxical fMRI overconnectivity upon neural silencing of fronto-cortical activity1Functional Neuroimaging Laboratory, Istituto Italiano di Tecnologia, Rovereto, Italy, 2Center for Mind and Brain Sciences, University of Trento, Rovereto, Italy, 3Neuronal Computational Laboratory, Istituto Italiano di Tecnologia, Rovereto, Italy, 4Biology Department, University of Pisa, Pisa, Italy, 5Systems Neurobiology Laboratory, Istituto Italiano di Tecnologia, Rovereto, Italy
Synopsis
Neuroimaging measurements of functional connectivity are commonly interpreted as an index of reciprocal interareal communication. However, direct testing of this hypothesis has been lacking. Using chemogenetics, electrophysiology and resting-state fMRI in the mouse, we show that acute and chronic silencing of the prefrontal cortex result in paradoxical rsfMRI overconnectivity of the mouse default mode network (DMN) and increased delta activity, an effect relayed to wider cortical territories by polymodal thalamic areas. Our results challenge prevailing interpretations of functional connectivity and implicate a critical contribution of sub-cortical rhythm generators to the establishment of large-scale functional coupling.
Introduction
Spatiotemporal correlations in the resting-state fMRI (rsfMRI) signal are commonly interpreted as an index of reciprocal interareal communication and functional connectivity. This notion is corroborated by the observation that functionally-synchronous brain regions are reciprocally interconnected by direct anatomical links, and that rsfMRI network topography closely recapitulates spatial patterns of anatomical connectivity1,2. Computational modelling supports this view, as synchronous rsfMRI fluctuations can be modelled via the intersection of local dynamic processes and reciprocal long-range neuronal interactions3. However, despite theoretical support for this framework, structurally based models of rsfMRI connectivity have not yet been comprehensively validated through experimental manipulations in the living brain. The combined use of rsfMRI and DREADD-based chemogenetics (chemo-fMRI4) offers the opportunity to bridge this knowledge gap, enabling a disambiguation of the correlative nature of rsfMRI coupling. Using this approach, Grayson et al., reported that neural silencing of the amygdala in primates results in disrupted rsfMRI activity in cortical areas anatomically linked to this region, substantiating prevailing interpretations of rsfMRI connectivity as an index of direct interareal communication5. However, clinical research shows that multiple neurological disorders entailing a physical disruption of cortical nodes (e.g. Parkinson’s, multiple sclerosis, etc.) are prominently associated with increased interareal rsfMRI connectivity6. These observations suggest that complementary region or state-dependent synchronizing mechanisms may contribute to brain-wide rsfMRI coupling and call for a deeper investigation of the fundamental relationship linking regional activity to macroscale functional connectivity. Using chemogenetics, electrophysiology and rsfMRI in the mouse7,8, here we show that neural inhibition of the prefrontal cortex result in paradoxical overconnectivity of the mouse default mode network (DMN) and increased delta activity, an effect relayed to wider cortical territories by polymodal thalamic areas. These results challenge prevailing interpretations of functional connectivity and implicate a critical contribution of sub-cortical rhythm generators to the establishment of large-scale functional coupling.Methods
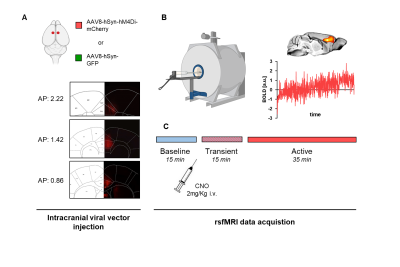
An overview of experimental procedures is provided in Figure 1. We used chemogenetics to obtain a sustained pan-neuronal silencing of the mouse anterior cingulate cortex (here referred to as prefrontal cortex – PFC), a core component of the rodent DMN and hub region of the mouse brain. The PFC was bilaterally injected with a viral vector expressing the inhibitory DREADD receptor hM4Di (or GFP) in adult male C57BL6 mice (hSyn-hM4D(Gi), n=15, hSyn-GFP control, n=19). rsfMRI connectivity was mapped under light sedation before and after chemogenetic silencing of the PFC, obtained upon intravenous injection of CNO (2 mg/kg). Electrophysiological recordings were carried out hM4Di expressing (n=5) and control GFP mice (n=5) under the same experimental conditions of rsfMRI. Chronic inhibition of the mouse PFC was obtained via overexpression of an inward rectifying potassium channel (n=16 hSyn-E224G, and n=19 hSyn-GFP).Results and discussion
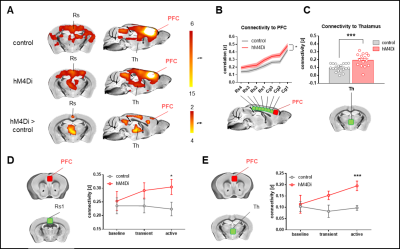
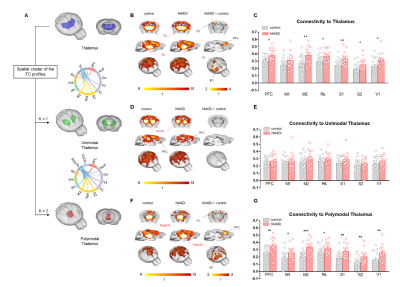
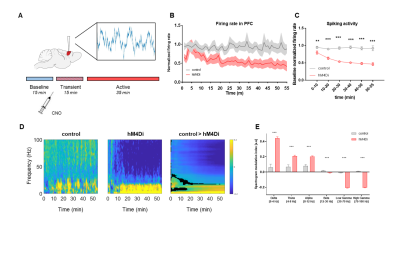
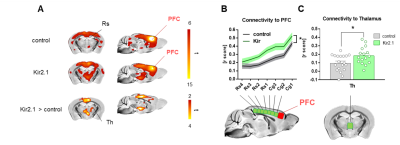
Chemogenetic silencing of the prefrontal cortex (PFC) elicited region-specific functional connectivity increases in several components of the mouse DMN, with a prominent involvement of the posterior cingulate/retrosplenial cortex and mediodorsal thalamus (Fig. 2). The effect reflected a gradual increase in rsfMRI coupling of each of these substrates with the PFC upon the injection of CNO. Interestingly, PFC silencing was associated with an altered topography of thalamic co-activation patterns of infraslow activity as measured by Gutierrez-Barragan et al9. This observation prompted us to probe a role for the thalamus in the observed fMRI oversynchronization (Fig. 3). By clustering the temporal profile of thalamo-frontal rsfMRI timecourses using the k-means algorithm, we identified two thalamic sub-territories corresponding to a midline “polymodal” and two “unimodal” lateral sub-divisions. We next probed the functional connectivity of each of these subdivisions, or the thalamus as a whole, found that polymodal, but not unimodal thalamus, was overconnected to widespread cortical territories extending beyond the PFC, suggesting a relay function of this structure to extra prefrontal areas. Importantly, CNO administration robustly reduced spontaneous firing rate in hM4Di expressing mice but not in control animals (Fig 4). Consistent with the observed rsfMRI hyper-synchronization, this effect was associated with a robust reduction in gamma power and increase slow-wave and delta activity. Finally, to further corroborate these findings, we induced in a separate cohort of mice a non-reversible chronic inhibition of the PFC via viral over-expression of an inward-rectifier potassium channel. rsfMRI mapping in these animals recapitulated the same over-connectivity profile obtained acutely with acute chemogenetic manipulations (Fig. 5). This result extends our findings, rules out unspecific effects of CNO, and suggests that the overconnected states cannot be homeostatically compensated, pointing at a possible pathological or translational relevance of our findings for brain disorders characterized by regional imbalances in cortical output.Conclusion
Our results demonstrate that acute and chronic neural silencing of associative cortical regions result in a paradoxical rsfMRI hyper-synchronization. The observed breakdown between cortical output and rsfMRI synchronization challenges prevailing (i.e. structurally-based) interpretations of functional connectivity, and implicates a critical contribution of sub-cortical rhythm generators to the establishment of brain-wide functional coupling.Acknowledgements
This work was supported by the Brain and Behavior Foundation (NARSAD; Independent investigator Grant; no. 25861) and the European Research Council (ERC - DISCONN, GA802371).References
1. J. Goni et al., Resting-brain functional connectivity predicted by analytic measures of network communication. Proceedings of the National Academy of Sciences 111, 833-838 (2013).
2. C. J. Honey et al., Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences 106, 2035-2040 (2009).
3. A. Ponce-Alvarez et al., Resting-State Temporal Synchronization Networks Emerge from Connectivity Topology and Heterogeneity. PLoS Comput Biol 11, e1004100 (2015).
4. A. Giorgi et al., Brainwide mapping of endogenous serotonergic transmission via chemogenetic-fMRI. Cell Reports, (2017).
5. D. S. Grayson et al., The Rhesus Monkey Connectome Predicts Disrupted Functional Networks Resulting from Pharmacogenetic Inactivation of the Amygdala. Neuron 91, 453-466 (2016).
6. F. G. Hillary, J. H. Grafman, Injured Brains and Adaptive Networks: The Benefits and Costs of Hyperconnectivity. Trends in Cognitive Sciences 21, 385-401 (2017).
7. F. Sforazzini, A. J. Schwarz, A. Galbusera, A. Bifone, A. Gozzi, Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. Neuroimage 87, 403-415 (2014).
8. A. Liska, A. Galbusera, A. J. Schwarz, A. Gozzi, Functional connectivity hubs of the mouse brain. Neuroimage 115, 281-291 (2015).
9. D. Gutierrez-Barragan, M. A. Basson, S. Panzeri, A. Gozzi, Infraslow State Fluctuations Govern Spontaneous fMRI Network Dynamics. Current Biology 29, 2295-2306.e2295 (2019).
Figures