1115
Effect of inhibitory neural activities to BOLD fMRI1Center for Neuroscience Imaging Research (CNIR), Institute for Basic Science (IBS), Suwon, Korea, Republic of, 2Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of
Synopsis
BOLD fMRI combined with optogenetics allows for brain-wide neural network studies. Most studies have focused on activity of excitatory neurons, which is presumably to contribute BOLD fMRI dominantly. However, fMRI response evoked by inhibitory neural activities is unknown. Here, we investigated 15.2T BOLD response of optogenetically stimulated GABAergic neural activation, and verified the results with electrophysiology.
Introduction
BOLD fMRI combined with optogenetics has revealed functional networks in the rodent brain by targeting excitatory neurons1-3. However, BOLD response induced by inhibitory neural activities remains unclear1, even though the effect of inhibitory neurons on hemodynamic response has been investigated by optical imaging with optogenetics4-7. Since inhibitory and excitatory neurons are networked closely8, it has been difficult to separate the response of inhibitory neurons from excitatory neurons in the cortex. Here, we investigated BOLD response with optogenetic stimulation on GABAergic neurons in the cortex of the mouse brain, then verified with neural recording for interpretation of fMRI data.Methods
Inhibitory neuron-specific optogenetic VGAT-ChR2-EYFP transgenic mice (20g-27g) were used in electrophysiology (n=3) and fMRI experiment (n=5). For control experiment, wild type mice (n=3, 21g-25g) were used in fMRI experiment. An optical fiber (d=105μm) was implanted in the primary somatosensory forelimb area (S1FL) for optical stimulation. For anesthesia, ketamine and xylazine cocktail were intraperitoneally injected for anesthesia induction (100mg/kg and 10mg/kg, respectively) and supplementary anesthesia during experiment intermittently (25mg/kg and 1.25mg/kg, respectively)9.Optical stimulation (473nm, 70mW/mm2) was given to S1FL with stimulation parameters of 20Hz frequency with 10ms pulse width or 1Hz with 200ms pulse width, and 20% duty cycle. Note that the total amount of light power deposition is same. All trials consisted of 40s(baseline)-20s(stimulation)-60s(rest)-20s(stimulation)-60s(rest).
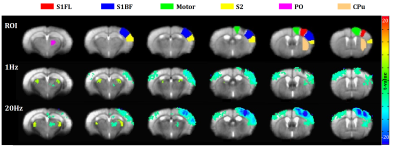
BOLD-fMRI data were acquired on 15.2T Bruker BioSpec scanner with single-shot GE-EPI with TR/TE=1000/11.5ms, FA=50° and spatial resolution=156x156x500µm3. In order to generate a standardized brain template, all EPI images from experimental group and control group (n=8) were averaged and co-registered10. Six ROIs which are located in ipsilateral side of optical fiber were defined based on Allen mouse brain atlas (Fig. 1). Peak amplitude and peak time were measured within 40s after stimulation onset.
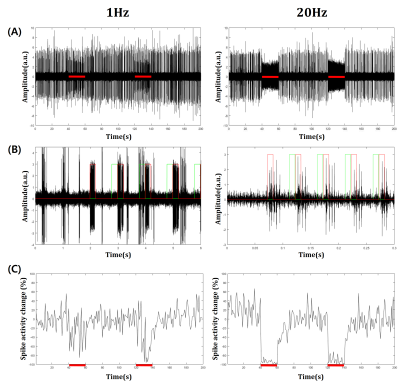
Electrophysiology data were acquired using a 16-channel optrode for simultaneous neural recording and optical stimulation. An optrode was implanted in S1FL confirmed by CBV-weighted optical intrinsic signal imaging with electrical forepaw stimulation. Multi-unit activity (MUA) data were acquired from filtered raw data (bandpass, 300Hz-3000Hz), then spike detection was performed11. Spontaneous spike activity (as spikes per second) was calculated during resting periods and only in the inter-stimulus durations (Fig. 3B, green lines) during stimulation periods (20s) for excluding stimulation-induced inhibitory activities.
Results
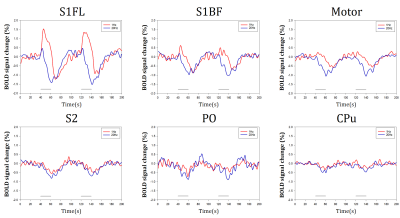
Optical stimulation of inhibitory neurons in S1FL resulted in negative BOLD responses in the regions which organize somatosensory networks (Fig. 1), as reported in a previous fMRI study12. In order to analyze response in the stimulation site (S1FL) and other regions, we extracted time courses from each ROI (Fig. 2). Response in S1FL was multi-phasic and showed dependence on the stimulation frequency: Positive peak amplitude was significantly higher with 1Hz stimulation (1.80±0.40% for 1Hz; 0.69±0.26% for 20Hz) while negative peak amplitudes were not much different (-1.55±0.54% for 1Hz; -1.68±0.29% for 20Hz). In addition, peak time of negative response was slower with 1Hz (32.20±2.83s) than 20Hz (23.10±1.70s). Responses in S1BF and motor cortex presented frequency dependence in terms of amplitude and phase, while S2, PO and CPu showed difference only in amplitude (Fig. 2). No significant responses were detected in the control group by optical stimulation (not shown here).To investigate the source of BOLD response, multi-unit recording was performed. Representative MUA data from a mouse demonstrated that 20Hz stimulation effectively inhibited spontaneous spike activity while 1Hz did not suppress effectively (Fig. 3A). However, putative inhibitory neuronal spikes which are distinct from spontaneous spikes appeared during stimulation robustly (Fig. 3B, red lines). Thus, to quantify suppression effect, we extracted only the spontaneous excitatory activity by analyzing the signals that appeared just before the stimulation onset (Fig. 3B, green lines). Spontaneous spike activity was mostly suppressed in 20Hz, but less in 1Hz during stimulation periods (-30.93±12.43% for 1Hz; -94.71±3.91% for 20Hz) (Fig. 3C).
Discussion & Conclusion
In the stimulation site, BOLD responses induced by inhibitory neural activities showed frequency dependence; 1Hz response has an initial higher positive response than 20Hz response. This biphasic response may be due to the intrinsic hemodynamic properties by sub-type inhibitory neural activities7, and inhibition/excitation imbalance. Negative BOLD responses were observed in the functionally connected regions. This is likely due to reduced projection of spontaneous activities from the stimulation site by the reduction/suppression of excitatory neural activities in S1FL.Frequency-dependent BOLD responses in S1FL can be explained by electrophysiogical data (Fig. 3). 1Hz optogenetic stimulation activates inhibitory neurons but induces ineffective suppression on spontaneous activities, while 20Hz stimulation causes complete inhibition of spontaneous activities. Thus, it is presumed that the BOLD response in 1Hz stimulation represents more intrinsic inhibitory neuronal activities, while 20Hz has a combined response from inhibitory and suppressed excitatory neuronal activities13. Based on two different frequency data, we can speculate that the BOLD response of inhibitory neural activities has rapid peak time (9.30±2.75s for 1Hz stimulation), which is faster than that of excitatory neural activities12,14. A small positive peak in 20Hz BOLD is likely due to fast inhibitory and slow excitatory hemodynamic response. Further studies are needed for investigating hemodynamic responses of excitatory vs. inhibitory activities.
Acknowledgements
This work was supported by IBS-R015-D1.References
1. Lee JH et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788-792.
2. Weitz AJ et al. Optogenetic fMRI reveals distinct, frequency-dependent networks recruited by dorsal and intermediate hippocampus stimulations. Neuroimage. 2015;107:229-241.
3. Leong ATL et al. Optogenetic fMRI interrogation of brain-wide central vestibular pathways. Proc Natl Acad Sci USA. 2019;116:10112-10129.
4. Urban A, Rancillac A, Martinez L and Rossier J. Deciphering the neuronal circuitry controlling local blood flow in the cerebral cortex with optogenetics in PV::Cre transgenic mice. Front Pharmacol. 2012;3:105.
5. Vazquez AL, Fukuda M and Kim S-G. Inhibitory Neuron Activity Contributions to Hemodynamic Responses and Metabolic Load Examined Using an Inhibitory Optogenetic Mouse Model. Cereb Cortex. 2018;28:4105-4119.
6. Desjardins M et al. Awake Mouse Imaging: From Two-Photon Microscopy to Blood Oxygen Level–Dependent Functional Magnetic Resonance Imaging. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(6):553-542.
7. Krawchuk MB, Ruff CF, Yang X, Ross SE and Vazquez AL. Optogenetic assessment of VIP, PV, SOM and NOS inhibitory neuron activity and cerebral blood flow regulation in mouse somato-sensory cortex. J Cereb Blood Flow Metab. 2019.
8. Meyer HS et al. Inhibitory interneurons in a cortical column form hot zones of inhibition in layers 2 and 5A. Proc Natl Acad Sci USA. 2011;108:16807–16812.
9. Shim H-J et al. Mouse fMRI under ketamine and xylazine anesthesia: Robust contralateral somatosensory cortex activation in response to forepaw stimulation. Neuroimage. 2018;177:30-44.
10. Nie B et al. A stereotaxic MRI template set of mouse brain with fine sub-anatomical delineations: Application to MEMRI studies of 5XFAD mice. Magn Reson Imaging. 2019;57:83-94.
11. Rey HG, Pedreira C and Quian Quiroga R. Past, present and future of spike sorting techniques. Brain Res Bull. 2015;119:106-117.
12. Jung WB, Shim H-J and Kim S-G. Mouse BOLD fMRI at ultrahigh field detects somatosensory networks including thalamic nuclei. Neuroimage. 2019;195:203-214.
13. Boorman L et al. Negative Blood Oxygen Level Dependence in the Rat:A Model for Investigating the Role of Suppression in Neurovascular Coupling. J Neurosci. 2010;30(12):4285-4294.
14. Iordanova B, Vazquez AL, Poplawsky AJ, Fukuda M and Kim S-G. Neural and hemodynamic responses to optogenetic and sensory stimulation in the rat somatosensory cortex. J Cereb Blood Flow Metab. 2015;35(6):922-932.
Figures