1103
Cross species validation of the layer-fMRI VASO contrast mechanism: data comparison against pre-clinical 2D-OIS and CBV-MRI gold standards.
Aneurin J Kennerley1, Benedikt A Poser2, Frida H Torkelsen1, Rainer Goebel2, Amanda Kaas2, and Laurentius Huber2
1Chemistry, University of York, York, United Kingdom, 2Maastricht Brain Imaging Centre, Maastricht University, Maastricht, Netherlands
1Chemistry, University of York, York, United Kingdom, 2Maastricht Brain Imaging Centre, Maastricht University, Maastricht, Netherlands
Synopsis
With recent advances in ultra-high-field MRI hardware and sequence mechanisms, it has become possible to capture CBV-weighted fMRI signal across cortical layers. However, the exact contrast mechanisms of layer-dependent VASO has not been fully validated with gold-standard pre-clinical methods.
Introduction
Methodological advances in functional Magnetic Resonance Imaging (fMRI) in recent years now allow researchers to approach the mesoscopic spatial regime of cortical layers and cortical columns1. The cerebral blood volume (CBV) sensitivity, and improved spatial specificity, offered by the non-invasive vascular space occupancy (VASO) contrast mechanism, which can be easily implemented on existing clinical MRI hardware, promises an unprecedented non-invasive glimpse into the laminar circuits of the human brain. At clinical field strengths (<3T) the VASO contrast relies the difference in blood and tissue T1 times. The MR signal of blood compartment can be nulled (using an inversion recovery approach) and functional changes in the blood volume can be deduced from the complementary signal changes in the measured tissue compartment. VASO is particularly attractive at high fields (7 T) due to the increase in image SNR and the longer longitudinal relaxation time of blood, which can amplify VASO's functional T1-contrast2. However, to date it has not been conclusively established to what extent macrovascular contaminations might contribute to layer-dependent CBV fMRI results and thus wider uptake of the VASO fMRI method is compromised.The goal of this current study was to utilise a multi-modal invasive pre-clinical rodent model to investigate cortical surface macrovascular contamination as a possible confound to the VASO contrast. We compared functional changes (in response to somatosensory stimulation of the whisker pad) measured with VASO and Blood Oxygenation Level Dependent (BOLD) fMRI, to high spatial and temporal resolution concurrent 2D optical imaging spectroscopy (2D-OIS) measurements of total and deoxygenated haemoglobin (HbT and Hbr respectively) to validate the VASO CBV signal source. MION contrast agent based measurement of the CBV pool, within the same animals and scanning sessions offered further validation of the layer-dependent CBV profiles measured with VASO-fMRI. Ultimately, we sought to compare layer-dependent fMRI profiles of CBV and BOLD across rodents and humans in with the same non-invasive SS-SI-VASO2 sequence in similar areas of somatosensory cortex.
Methods
Human MRI: Multiple human 12-min acquisitions were conducted in n=7 participants for passive stroking and finger tapping tasks. Human data acquisition was approved by the Maastricht ethical review board: ERCPN:180_03_06_2017 and the NIH Combined Neuroscience Institutional Review Board-approved protocol (93-M-0170, ClinicalTrials.gov identifier: NCT00001360). Functional data of GE-BOLD and cerebral blood volume (CBV) were concomitantly acquired with SS-SI-VASO. fMRI readout parameters were: in-plane resolution 0.7mm, slice-thickness: 1.2-1,8 mm perpendicular to the cortex, TE=25ms, in-plane PF=6/8 with POCS8, FLASH-GRAPPA=1, TR=1.6+1.6s, 3D-EPI readout3, ‘classic’ 7T Magnetom (Siemens Healthineers), 32-ch NOVA coil, SC72 body gradient.Rodent MRI: All aspects of these methods and their development were performed with UK Home Office approval under the Animals (Scientific Procedures) Act 1986. Measurements were made on a 7T MRI system (Bruker BioSpec, 310mm bore). Urethane anaesthetized animals (n=4) were artificially ventilated and cannulated for monitoring arterial blood pressure and intravenous infusion. A thinned skull cranial window allowed direct optical imaging of the cortex (see below). fMRI measurements (TE = 13ms, 0.5x0.5x3mm) of BOLD and CBV signal changes were obtained with concurrent optical measurements of HbT and blood oxygen saturation changes4-6. For direct comparison to VASO results, contrast agent measured CBV-MRI was performed (MION 8 mg/kg) with the optical data used as a standard. Signal changes were corrected for BOLD contamination.
Rodent 2D-OIS: Intrinsic optical imaging of the underlying haemodynamics within the magnet bore utilised a non-magnetic endoscope connected to a switching Galvanometer (Four λ’s = 495, 586, 559 and 575nm) and a CCD camera (32Hz frame rate)5. Spectral analysis was based Beer-Lambert law and incorporated a MR based heterogeneous tissue model6; producing 2D images over time, of oxy-, deoxy- and total haemoglobin changes (HbO2, Hbr and HbT respectively).
Results and Discussions
We find that in the rodent model both the BOLD fMRI signal and 2D-OIS measures of blood oxygenation are sensitive to large pial veins. The location of large pail vein signal matches with the location of the respective fMRI contrasts (Fig 1.). However, we also see evidence of layer-unspecific CBV signal changes at the location of large pial arteries. We find that the layer-dependent layer-profiles are the same (within error) for VASO-fMRI and MION-fMRI (Fig. 2). Note that MION and VASO are inherently measured in different physical units. VASO is measured in [ΔCBV ml / 100 ml of tissue], whereas MION is measured in [ΔCBV / CBVrest]. For a fair comparison, the local distribution of CBVrest needs to be taken into account. CBV sensitive layer fMRI responses in the primary somatosensory cortex are comparable in shape across species (Fig. 3). We find that passive touch of fingers in awake humans results in three times larger VASO signal change compared to electrical whisker pad stimulation in anaesthetised rodents.Conclusion
We conclude that the layer-dependent CBV change measured with non-invasive VASO methods reveals highly localized activity changes (independent of large veins), as expected from established pre-clinical imaging modalities of OIS and MION-fMRI. The confirmation that the VASO contrast is indeed a reliable estimate of layer-specific CBV changes is very valuable in human neuro-imaging: This validation increases the applicability of human layer-dependent fMRI results for neuronal interpretation in neuroscience studies7-8.Acknowledgements
Laurentius Huber and Aneurin Kennerley received funding from the York-Maastricht Partnership for this project. Laurentius Huber is funded form the NWO VENI project 016.Veni.198.032. Benedikt Poser received funding from R01 MH111444/MH/NIMH NIH and 16.Vidi.178.052. Rainer Goebel received funding from the European FET Flagship project ‘Human Brain Project’ (FP7-ICT-2013-FET-F/604102 Grant Agreements, No. 7202070 (SGA1) and No. 785907 (SGA2)).References
- Huber et al. (2017). “High-Resolution CBV-FMRI Allows Mapping of Laminar Activity and Connectivity of Cortical Input and Output in Human M1.” Neuron 96(6): 1253-1263.e7. https://doi.org/10.1016/j.neuron.2017.11.005.
- Huber et al. (2014) "Slab-selective, BOLD-corrected VASO at 7 T provides measures of cerebral blood volume reactivity with high signal-to-noise ratio". Magn. Reson. Med., 72 (2014), pp. 137-148, 10.1002/mrm.24916
- Poser et al. (2010). “Three Dimensional Echo-Planar Imaging at 7 Tesla.” NeuroImage 51(1): 261–66. http://dx.doi.org/10.1016/j.neuroimage.2010.01.108 (July 15, 2012).
- Kennerley et al. 2013. “Does VASO contrast really allow measurement of CBV at High Field (≥7T)? An in-vivo quantification using concurrent Optical Imaging Spectroscopy”, ISMRM, 21st. 0757.
- Kennerley et al. (2009). “Refinement of Optical Imaging Spectroscopy Algorithms Using Concurrent BOLD and CBV FMRI.” NeuroImage 47(4): 1608–19., p. 675.03.
- Kennerley et al. (2005). “Concurrent FMRI and Optical Measures for the Investigation of the Hemodynamic Response Function.” Magnetic Resonance in Medicine 54(2): 354–65.
- Yu et al., (2019). “Layer-Specific Activation of Sensory Input and Predictive Feedback in the Human Primary Somatosensory Cortex.” Science Advances 5(5): eaav9053.
- Finn et al., (2019). 2019. “Layer-Dependent Activity in Human Prefrontal Cortex during Working Memory.” Nature Neuroscience: ahead of print. http://dx.doi.org/10.1038/s41593-019-0487-z.
Figures

OIS and fMRI activation maps of four animals: Column 1: total FOV of the endoscope. Columns 2-3: OIS estimates of Y and CBV. Draining veins show strong changes in Y, distant from activated tissue (black arrows). CBV changes, however, are confined to activated tissue. Though, pial arteries are contributing to the CBV change (white arrows). Columns 4-5: Coronal slices fMRI slices. When large pial veins are within the acquired slice, significant BOLD signal change distance of the site of highest CBV change can be seen (black arrows).
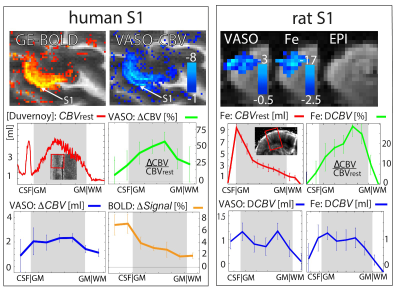
Comparison of CBV layer profiles in the primary sensory cortex in humans and rodents with VASO. It can be seen that the CBV layer profile in units of ΔCBV[%] is dominated from layer 4. When the same data are evaluated as ΔCBV [ml / 100ml of tissue], an additional signal component at the superficial layers becomes apparent. Only when VASO and Iron contrast agent based fMRI signals are analysed with the same physical units, the resulting layer-fMRI profiles are comparable.
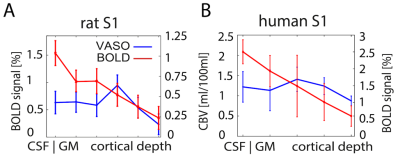
Cortical profiles of BOLD and VASO signal change in rat S1 (A) and human S1 (B): Data shown refer to averages across four rats and five human participants, respectively. Laminar-dependent profiles reveals different shapes for VASO and BOLD fMRI. BOLD signal change is highly dominated from surface laminae, while VASO peaks in middle cortical laminae, presumably reflecting thalamocor- tical input, as expected. Note the different scaling of the y-axis in panel A) and B).