1100
On the Feasibility of Noncontrast Valvular Cine MRI with High Spatial Resolution and High Frame Rate Using Deep-learning-powered Acceleration
Peng Lai1, Christopher M Sandino2, Shreyas S Vasanawala3, Anne Menini1, Haonan Wang4, Anja C.S Brau1, and Martin A Janich5
1GE Healthcare, Menlo Park, CA, United States, 2Electrical Engineering, Stanford University, Palo Alto, CA, United States, 3Radiology, Stanford University, Palo Alto, CA, United States, 4GE Healthcare, Waukesha, WI, United States, 5GE Healthcare, Munich, Germany
1GE Healthcare, Menlo Park, CA, United States, 2Electrical Engineering, Stanford University, Palo Alto, CA, United States, 3Radiology, Stanford University, Palo Alto, CA, United States, 4GE Healthcare, Waukesha, WI, United States, 5GE Healthcare, Munich, Germany
Synopsis
Valvular imaging is challenging to conventional cine MRI due to its requirement of very high spatial and temporal resolution. This work preliminarily investigated valvular cine MRI with highly accelerated data acquisition powered by deep learning reconstruction. Our results demonstrated the feasibility to resolve valve anatomy and motion with nearly 1mm spatial resolution and 10ms frame rate, while flow-induced dephasing generates shading in blood pool and can complicate valve visualization.
INTRODUCTION
Phase-contrast MRI enables quantitative measurement of blood velocity and thus assessment of valvular diseases showing abnormal flow (e.g. stenotic and regurgitant flow). Imaging valvular motion could further elucidate valvular pathologies to help guide management. However, resolving the thin rapidly moving leaflets necessitates imaging resolution and frame rate much beyond the acceleration capability of conventional clinical cardiac MRI. This work aims to investigate the feasibility of valvular imaging using highly accelerated cine MRI powered by deep-learning image reconstruction.METHODS
To achieve the high spatial and temporal resolution for valvular imaging, we modified a conventional cine sequence to perform variable density time-interleaved k-space sampling with an acceleration factor of 12. An unrolled reconstruction network [1] was trained on cardiac cine datasets in a supervised learning manner and used to reconstruct valvular cine images from sparse k-space data. The network consists of 8 cascades of two alternative modules, namely, a residual U-net to exploit spatiotemporal data prior and a MR physics module to enforce consistency with sampled data and coil sensitivity. To test feasibility, noncontrast tricuspid and mitral valve images were obtained in a 4-chamber view in high spatial and temporal resolution from 3 healthy volunteers on a GE 3T MR system. Data were acquired during breathholds using bSSFP and gradient echo (GRE) with and without flow compensation (FC). Imaging parameters are listed in Table 1. Readout resolution in bSSFP was lowered to shorten TR due to potential banding artifacts with long TR. A small arrhythmia rejection window (15%) was used to reduce motion blurring due to cardiac cycle variations. As a comparison, conventional bSSFP cine images were also collected in the same slices using ASSET acceleration of 2x.RESULTS
Figure 1 shows images collected in different acquisition modes. In bSSFP cine, depiction of the valves is compromised by reduced readout resolution (a) and flow artifacts originated from pulsatile aortic flow (b). GRE without FC (c) suffers from severe flow dephasing that creates dark artifacts in the blood pool and obscures the valve depiction. GRE with FC and readout along the ventricular long axis direction (e) provides the best valve visualization of the tricuspid valve, while imaging with 90° in-plane rotation (d) shows increased dephasing artifacts due to large uncompensated flow. However, the mitral valve is untraceable in all three cases due to shading in left atrial blood. Figure 2 shows the tricuspid valve at eight different cardiac phases (a-h), resolving the valvular opening and closing in a cardiac cycle. In comparison, conventional cine (i-l) barely captures the valve only at right-ventricular systole when the valve is fully closed.DISCUSSION
GRE is more robust to field inhomogeneity than bSSFP, but is sensitive to signal dephasing due to blood flow. The current sequence implements FC only along readout and so the readout direction is aligned with the long axis with dominant flow to minimize flow effects, whereas uncompensated lateral flow still produces remarkable signal dephasing. The difference in flow pattern and / or velocity in left atrium might have contributed to invisible mitral valve. Adding FC to both readout and phase encoding should more completely suppress flow-induced artifacts and improve valve visualization. As shown in Fig.1.f, lower flip angle produces reduced flow dephasing and more homogenous blood pool. However, its much lower SNR and blood-valve contrast makes the mitral valve indistinct. This result suggests that imaging with contrast agent can better visualize the mitral valve.CONCLUSION
Our preliminary experiments demonstrated that DL reconstruction enables the speed for resolving valve anatomy and motion in breathheld scans, which is infeasible with the conventional cine sequence. However, visualization of the valve, especially the mitral valve, is still complicated by blood flow induced signal dephasing. Future work will implement more complete FC and conduct further evaluations.Acknowledgements
No acknowledgement found.References
[1] Sandino CM, ISMRM 2019.Figures
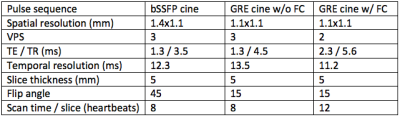
Table 1.
Imaging parameters
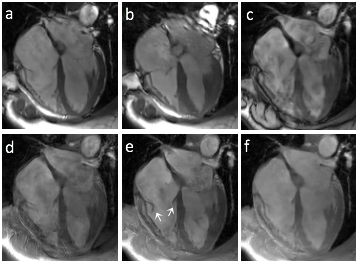
Figure 1.
Images acquired with bSSFP at 2 phases (a, b), GRE without FC
(c), GRE with FC and readout orthogonal to ventricular long axis (d), GRE with FC and
readout along ventricular long axis with flip angle of 15°
(e) and 10° (f).
White arrows in (e) indicate the tricuspid valve leaflets.
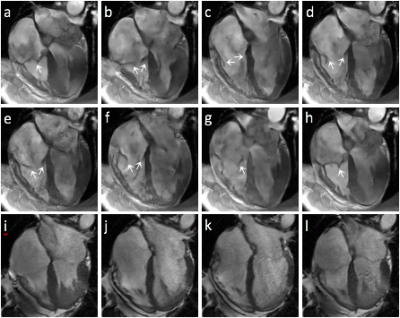
Figure 2. Valve images at 8 phases (a-h) acquired with the proposed
method and the conventional bSSFP cine (i-l) corresponding to the phases in b,
d, f and h, respectively. White arrows in (a-h) indicate the tricuspid valve
leaflets.