1088
Highly efficient respiratory motion-compensated 3D water/fat late gadolinium enhanced atrial wall imaging1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Cardiovascular Imaging Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4Cardiovascular MR Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
3D late gadolinium enhanced (LGE) imaging is a promising technique for the non-invasive assessment of atrial fibrosis. In order to minimize respiratory motion, current 3D LGE atrial imaging relies on diaphragmatic navigator gating, leading to time-consuming scans with unpredictable duration. Here we introduce a highly efficient respiratory motion-compensated 3D water/fat IR-prepared LGE atrial imaging protocol with predictable scan time. Preliminary results demonstrate that the proposed approach enables depiction of atrial scar comparable to conventional 3D atrial LGE imaging, but with a significantly shorter scan time of <5 minutes. The proposed approach holds promise for high-resolution atrial wall imaging.
INTRODUCTION
Atrial cardiac MR imaging with late gadolinium enhancement (LGE) is the only non-invasive tool for atrial substrate characterization in clinical use. Assessment of atrial morphology1 and fibrosis2 by 3D LGE imaging has shown to have predictive value in determining clinical outcome following atrial ablation. Further, targeting of fibrosis by ablation is an emerging technique which may improve patient outcomes.3 Moreover, 3D atrial wall LGE imaging has shown a relevant role in the quantification of radiofrequency ablation scar formation.4,5 Current 3D atrial LGE imaging protocols are commonly based on free-breathing diaphragmatic navigator-gated (dNAV) inversion recovery (IR)-prepared sequences. These approaches produce good-quality images in subjects with regular breathing patterns; however, large variations in respiratory efficiency between subjects lead in practice to highly time-consuming scans and often in poor image quality, due to residual motion artefacts and signal variations due to heart rate variability and/or gadolinium washout during prolonged acquisition times.6,7Here we introduce a highly efficient motion-compensated water/fat 3D LGE atrial wall imaging protocol, which integrates 2D coronal image navigators (iNAV)8 to enable 100% respiratory scan efficiency (no data rejection) and predictable scan time and utilizes an undersampled Cartesian variable-density spiral like trajectory9 to enable further acceleration. In this study, we tested our approach in a cohort of patients and compare its performance against conventional dNAV-based atrial LGE imaging.
METHODS
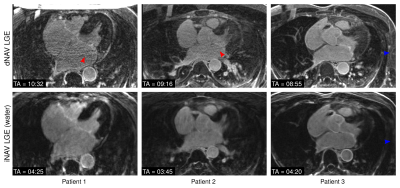
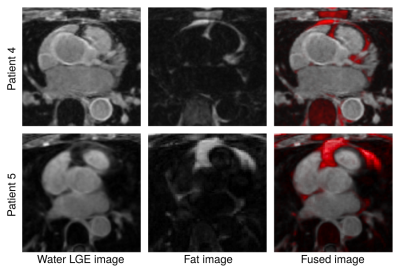
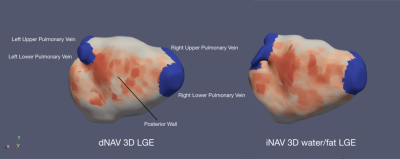
Image acquisition consists of an ECG-triggered dual-echo 3D inversion recovery (IR)-prepared spoiled gradient echo sequence (Figure 1a). 3D dual-echo data are acquired with an undersampled variable-density golden-step Cartesian trajectory with spiral profile order sampling.9 Coronal 2D iNAVs are acquired using low flip-angle excitation pulses before the 3D acquisition10 and are used to estimate superior-inferior (SI) and right-left (RL) motion by tracking a template around the left atria. The estimated SI & RL motion are used to correct the acquired 3D dual-echo data to a reference respiratory position by applying a shift in k-space. Motion-compensated dual-echo 3D data is reconstructed using HD-PROST11, and resulting images are used to compute the final 3D whole-heart water/fat LGE images using a 2-point Dixon water/fat separation algorithm12 (Figure 1b).Eight patients scheduled for first time atrial fibrillation ablation referred for cardiac MRI were recruited and scanned at a 1.5T system (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). The clinical protocol included standard dNAV 3D LGE imaging, starting 20 minutes after bolus injection of 0.2mmol/kg Gd-based contrast agent, with a fat-suppressed IR-prepared sequence. Relevant imaging parameters include transverse orientation, resolution 1.3×1.3×4mm3, interpolated to 1.3×1.3×2mm3 during image reconstruction, TR/TE=3.52/1.74ms, FA=20°, BW=365Hz/px, GRAPPA parallel imaging (2-fold undersampling, 24 reference lines). After conventional dNAV 3D LGE imaging, data were acquired with a prototype implementation of the proposed iNAV-based water/fat 3D LGE imaging sequence, with matching transverse orientation, resolution and field of view, and the following imaging parameters: TR/TE1/TE2=7.16/2.38/4.76ms, FA=20°, BW=495Hz/px, 3-fold undersampling (with respect to a fully-sampled Cartesian acquisition). For each patient, atrial shell images were generated for both the dNAV and iNAV LGE imaging approaches using the CEMRG software.13
RESULTS
The proposed iNAV-based LGE acquisition was successfully completed in all subjects, with an average scan time of 4.5±1.2min, significantly shorter (p<0.001) than conventional dNAV-based LGE imaging (10.1±2.6min). A comparable depiction of the atrial wall can be observed with both techniques (Figure 2); however, iNAV-based LGE resulted in improved fat suppression and reduced motion artefacts. Furthermore, the proposed iNAV-based technique offers a fully co-registered complementary fat image that may be useful for characterization of peri-atrial fat (Figure 3). Although the iNAV-based LGE images were acquired after the optimal post-contrast time (starting 32.8±5.6 minutes after Gd administration), good image quality enabled accurate depiction of the atria and segmentation of atrial scar (Figure 4).CONCLUSION
We propose a novel highly efficient 3D water/fat LGE atrial imaging protocol with predictable scan time. The technique incorporates all acquired data for image reconstruction and enables atrial LGE imaging from a short scan of less than 5 minutes. Preliminary results show that our technique results in good depiction of atrial scar comparable to conventional dNAV-based imaging, but in a predictable and shorter scan time, holding promise for high-resolution atrial wall imaging.Acknowledgements
This work was supported by the following grants: EPSRC 1) EP/P032311/1; 2) EP/P007619/1; 3) EP/P001009/1; and the Wellcome/EPSRC Centre for Medical Engineering (NS/A000049/1).References
1. Bisbal F, Guiu E, Calvo N, et al. Left atrial sphericity: A new method to assess atrial remodeling impact on the outcome of atrial fibrillation ablation. J Cardiovasc Electrophysiol 2013;24:752–9
2. Chubb H, Karim R, Mukherjee R, et al. A comprehensive multi-index cardiac magnetic resonance-guided assessment of atrial fibrillation substrate prior to ablation: prediction of long-term outcomes. J Cardiovasc Electrophysiol 2019;jce.14111.
3. Blandino A, Bianchi F, Grossi S, et al. Left Atrial Substrate Modification Targeting Low-Voltage Areas for Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. PACE - Pacing Clin Electrophysiol 2017;40:199–212.
4. Peters DC, Wylie J V., Hauser TH, et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: Initial experience. Radiology 2007;243:690–5.
5. Williams SE, Harrison J, Chubb H, et al. The Effect of Contact Force in Atrial Radiofrequency Ablation Electroanatomical, Cardiovascular Magnetic Resonance, and Histological Assessment in a Chronic Porcine Model. JACC Clin Electrophysiol. 2015;1:421–31.
6. Marrouche NF, Wilber D, Hindricks G, et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation. JAMA 2014;311:498.
7. Quail M, Grunseich K, Baldassarre LA, et al. Prognostic and functional implications of left atrial late gadolinium enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2019;21:2.
8. Henningsson M, Koken P, Stehning C, et al. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med 2012; 67:437–45
9. Bustin A, Ginami G, Cruz G, et al. Five-minute whole-heart coronary MRA with sub-millimeter isotropic resolution, 100% respiratory scan efficiency, and 3D-PROST reconstruction. Magn Reson Med 2019;81:102-115.
10. Kunze KP, Piccini D, Forman C, et al. 3D Whole-Heart Imaging with Orientation-Independent 2D Image Navigators. Soc Magn Reson Angiogr 31st Annu Int Conf. 2019. p. 25.
11. Bustin A, Lima da Cruz G, Jaubert O, et al. High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI. Magn Reson Med 2019;81:3705–3719.
12. Liu J, Peters DC, Drangova M. Method of B0 mapping with magnitude-based correction for bipolar two-point Dixon cardiac MRI. Magn Reson Med. 2017;78:1862–9.
13. Sim I. Razeghi O, Karim R, Chubb H, Whitaker J, O’Neill L, et al. Reproducibility of Atrial Fibrosis Assessment Using CMR Imaging and an Open Source Platform. JACC Cardiovasc Imaging; 2019.
Figures