1083
Quantification of carotid plaque compositon with Multi-contrast Atherosclerosis Characterization (MATCH) versus multisequence carotid MRI1Radiology and Nuclear medicine, CARIM School for Cardiovascular Diseases, Maastricht, Netherlands, 2Radiology and Nuclear medicine, Maastricht university Medical Centre, Maastricht, Netherlands, 3Cedars-Sinai Medical Center, Biomedical Imaging Research Institute, Los Angeles, CA, United States
Synopsis
Multisequence MRI protocol usually includes an
MP-RAGE sequence for the identification of plaque compositions. Multisequence MRI
has some limitations including long scan time and image mis-registration
errors. Multi-contrast Atherosclerosis Characterization (MATCH) was developed
to overcome the above limitations. Eighteen patients with ≥2 mm carotid plaques
underwent 3.0T carotid MRI including conventional multisequence and MATCH. For
the artery-based component detection, excellent agreement was obtained for
LRNC, substantial for IPH and slight agreement for calcifications. No
significant difference between MATCH and conventional MRI was shown in
measurement of volume of LRNC/IPH, IPH, calcifications, percentage wall volume
and normalized wall index.
Synopsis
Multisequence carotid MRI is commonly used to quantify plaque composition, but it has some disadvantages, i.e. long scan time and image mis-registration errors. Multi-contrast Atherosclerosis Characterization (MATCH) was developed to overcome these limitations. Eighteen patients with ≥2 mm carotid plaques underwent carotid multisequence and MATCH MRI. For the artery-based component detection, excellent agreement between MATCH and multisequence MRI was obtained for identification of LRNC, substantial for IPH and slight agreement for calcifications. No significant differences were shown in measurements of volume of LRNC, IPH, calcifications, percentage wall volume and normalized wall index.Introduction
Carotid plaques with a large lipid-rich necrotic core, intraplaque haemorrhage and a thin fibrous cap are associated with an increased stroke risk.1 Multisequence magnetic resonance imaging (MRI) is commonly used for characterization of carotid atherosclerotic,2 but it has some limitations, including long scan time and image mis-registration errors. Multi-contrast Atherosclerosis Characterization (MATCH) was developed to overcome these limitations.3Purpose
To compare MATCH with multisequence MRI for the characterization and quantification of carotid plaque components.Methods
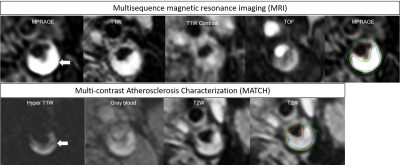
Eighteen symptomatic patients with ≥ 2 mm plaque underwent carotid multisequence and MATCH MRI on a 3.0T hybrid integrated PET-MRI scanner (Biography mMR, Siemens, Erlangen, Germany) using a dedicated radiofrequency coil. The sequence parameters of MATCH were as follows: repetition time/echo time (TR/TE): 10.1/4.35 msec, field of view (FOV): 160x160 mm, matrix size: 256x256, acquired in-plane resolution: 0.63x0.63 mm², slice thickness: 2 mm, number of slices: 18, flip angles: 8°,5°,10°, bandwidth: 130 Hz/pixel. Multisequence MRI (black blood pre- and post-contrast T1w TSE, time of flight (TOF) and magnetization prepared rapid acquisition gradient echo (MP-RAGE); acquired in-plane resolution: 0.6 x 0.6 mm²) was acquired according to recent expert consensus recommendations.2 One trained observer delineated the main plaque components (inner and outer vessel wall, intraplaque haemorrhage, lipid-rich necrotic core, and calcifications) on the multisequence and MATCH images. Image analysis of the MATCH images was performed independent of the multisequence images in a blinded way. Images quality was scored on a 5 point scale based on signal-to-noise ratio and visibility of vessel wall and substructures (1, poor and 5, excellent).4 Artery-based agreement in the detection of individual components by the two protocols was determined using a Cohen’s kappa test. Kappa values from 0 to 0.2 indicated slight agreement, 0.21 to 0.4 fair agreement, 0.41 to 0.60 moderate agreement, 0.61 to 0.8 substantial agreement, and >0.8 excellent agreement. The differences in outer wall volume, normalized wall index (NWI), and volume of plaque components between MATCH and the multisequence protocol were evaluated by Bland-Altman analysis and a paired t-test.Results
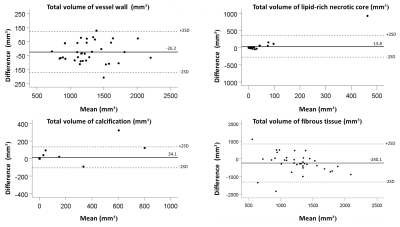
One total or nearly occluded artery was excluded. Thus, thirty-five carotid arteries were analysed. The mean quality scores of the MATCH images were lower than that of the multisequence images(p< 0.01). The scan time for MATCH and multisequence MRI was 4:44 and 10:84 minutes, respectively. Excellent agreement between the two protocols was obtained for identification of LRNC (k = 0.82), substantial for IPH (k= 0.768) (Fig.1) and slight agreement for calcifications (k= 0.389) (Table 1). No significant differences between MATCH and multisequence MRI were found in volume of LRNC (61.7±193.6 mm³ versus 47.8±153.7 mm³; P=0.182), IPH (66.7±261.5 mm³ versus 26.95±102.56 mm³; P=0.334), calcifications (45.9±156.09 mm³ versus 11.77±14.51 mm³; P=0.202), percentage wall volume (PWV) (54.8±9.1% versus 56.9±7.7 %; P=0.134) and NWI (0.15±0.25 versus 0.21±0.22; P=0.169). There was a small but significant difference in total volume of vessel wall (1269.07±333.89 mm³ versus 1295.32±329.81 mm³; P=0.041) and total volume of fibrous tissue (1131.4±370.31 mm³ versus 1371.6±502.04 mm³; P=0.013).Discussion
MATCH provides three different contrast weightings using a single sequence with a short scan time and without mis-registration errors, which makes it easy to implement in clinical practice.Conclusion
We demonstrated excellent to substantial agreement between MATCH and multisequence MRI for the identification of LRNC and IPH. There was only slight agreement for scoring presence of calcifications. Our study showed no significant differences between MATCH and multisequence sequences in volumes of all plaque components, expect for fibrous tissue and total vessel volume. Although the MATCH images have as a lower mean image quality score, short scan time and perfect co-registration are major advantages of MATCH.Acknowledgements
This research was supported by Stichting De Weijerhorst and by NWO, grant number VidW,1154.18.021References
1. Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI and Sanelli PC. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013;44:3071-7.
2. Saba L, Yuan C, Hatsukami TS, Balu N, Qiao Y, DeMarco JK, Saam T, Moody AR, Li D, Matouk CC, Johnson MH, Jager HR, Mossa-Basha M, Kooi ME, Fan Z, Saloner D, Wintermark M, Mikulis DJ, Wasserman BA and Vessel Wall Imaging Study Group of the American Society of N. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2018;39:E9-E31.
3. Fan Z, Yu W, Xie Y, Dong L, Yang L, Wang Z, Conte AH, Bi X, An J, Zhang T, Laub G, Shah PK, Zhang Z and Li D. Multi-contrast atherosclerosis characterization (MATCH) of carotid plaque with a single 5-min scan: technical development and clinical feasibility. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2014;16:53.
4. Yuan C, Kerwin WS, Yarnykh VL, Cai J, Saam T, Chu B, Takaya N, Ferguson MS, Underhill H, Xu D, Liu F and Hatsukami TS. MRI of atherosclerosis in clinical trials. NMR in biomedicine. 2006;19:636-54.
Figures