1082
3D Free-breathing Cardiac Magnetic Resonance Fingerprinting1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Philips Healthcare, Guildford, United Kingdom, 3Philips Research Hamburg, Hamburg, Germany
Synopsis
2D cardiac Magnetic Resonance Fingerprinting (cMRF) has been proposed for simultaneous and co-registered T1/T2 mapping using ECG-triggering and breath-holding. However, 2D cMRF provides limited coverage of the heart and is sensitive to residual through-plane respiratory motion. Here we propose respiratory motion-compensated 3D cMRF to enable whole-heart myocardial T1/T2 mapping in a single free-breathing scan. Respiratory bellows driven localized autofocus is proposed for beat-to-beat translational motion correction and patch-based low rank MRF reconstruction is employed to minimise residual aliasing. 3D cMRF enabled whole-heart T1/T2 mapping in ~7min scan time with comparable map quality to conventional 2D MOLLI, SASHA and T2-GraSE.
INTRODUCTION:
Multi-parametric mapping can improve myocardial tissue characterization.1 With conventional approaches multi-parametric mapping requires sequential acquisitions under several breath-holds, often leading to non-registered maps. 2D cardiac MR fingerprinting2 (cMRF) has been proposed to enable simultaneous and co-registered T1/T2 maps in a single breath-hold. However, 2D cMRF provides limited coverage of the heart, has limited signal-to-noise ratio (SNR) and can be affected by residual through-plane motion. Here we propose respiratory motion-compensated 3D cMRF to enable whole-heart myocardial T1 and T2 mapping in a single free-breathing scan. Variable inversion recovery (IR) and T2 preparation (T2prep) modules are used for parametric encoding, respiratory bellows drive an autofocus algorithm for respiratory motion correction and a subspace regularized reconstruction is employed to reduce scan time. The proposed 3D cMRF approach was evaluated in a standardized T1/T2 phantom and in seven healthy subjects in comparison to conventional 2D MOLLI, SASHA and T2-GraSE mapping techniques.METHODS:
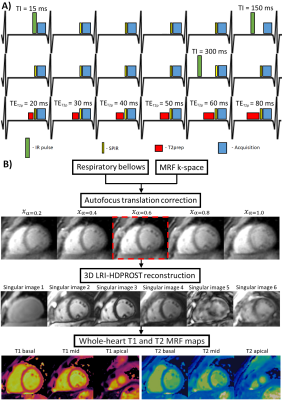
Acquisition: The proposed 3D cMRF uses a free-breathing, ECG-triggered acquisition with a stack of variable density spirals and different preparation pulses (inversion recovery-IR, T2prep and fat saturation via spectral presaturation with IR) applied in different heartbeats, as shown in Fig.1a. Motion compensation: Respiratory bellows were employed to obtain a (relative) 1D respiratory signal $$$r(t)$$$ and used to drive a localized autofocus algorithm3 for beat-to-beat translation correction. A set of $$$\alpha r(t)$$$ trial motion signals were used to motion correct and reconstruct a set of images $$$x_\alpha$$$. The optimal scaling $$$\hat{\alpha}$$$ was found by minimizing the localized gradient entropy $$$H(x_\alpha) = - \sum_i h_\alpha(x_\alpha(i))log_2h_\alpha(x_\alpha(i))$$$, where $$$h_\alpha$$$ is the normalized spatial gradient and $$$x_\alpha(i)$$$ is the i-th pixel intensity. The autofocus estimated beat-to-beat translational motion of the heart,$$$\hat{\alpha}r(t)$$$, was used to correct the acquired k-space data (Fig.1b). Reconstruction: LRI4 (Low Rank Inversion) - HDPROST5 (high dimensional patch-based reconstruction) is employed to reconstruct the motion corrected 3D cMRF data. LRI-HDPROST is formulated as: $$$\bf{x} = argmin_x \parallel \bf{AU_rFCx-k} \parallel _2^2 + \lambda \sum_b \parallel \bf{T_bx} \parallel _*$$$, where $$$A$$$, $$$U_r$$$, $$$F$$$ and $$$C$$$ are sampling, temporal compression (obtained from a truncated singular value decomposition of the MRF dictionary), non-uniform Fast Fourier Transform and coil sensitivity operators; $$$x$$$ are the temporally compressed singular images and $$$k'$$$ is the motion corrected k-space, whereas $$$T_b$$$ constructs 3D local tensor around each voxel b in the image by concatenating local (within a patch), non-local (between similar patches) and contrast (along the singular value domain) voxels along each dimension. T1 and T2 maps were obtained via inner product in the singular domain.EXPERIMENTS:
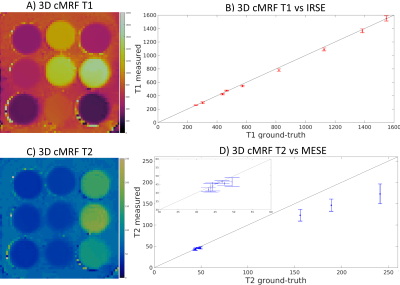
A standardised phantom and seven healthy subjects (3 female, 29±2 years) were scanned at 1.5T (Philips Ingenia). Key parameters included: field of view (FOV) = 352x352x120 mm3; resolution = 2x2x8 mm3; 19 slices; TE/TR = 1.25/6.80; gradient echo; 540 time-points per slice; 6-10º sinusoidally varying flip angle; 4s recovery between slice-encodings; acquisition time = 7 minutes. In phantom, 3D cMRF was compared against 2D inversion-recovery spin-echo (IRSE) and multi-echo spin-echo (MESE) for T1 and T2, respectively; whereas in vivo, 3D cMRF was compared with 2D MOLLI, SASHA and T2-GraSE conventional techniques.RESULTS:
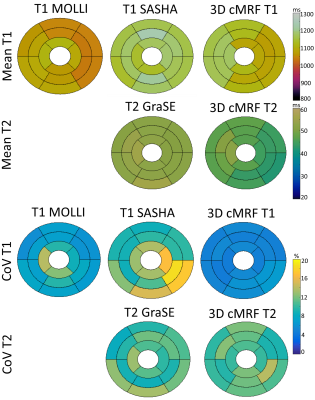
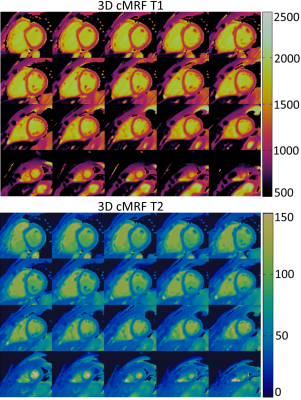
Phantom results show good agreement between proposed 3D cMRF and reference SE measurements. 3D cMRF in phantom achieved small errors in T1 and short T2 (<100 ms) of 3.2% and 3.5%, respectively, with slight underestimation in both T1 and T2 (Fig. 2). In vivo, the proposed 3D cMRF produced comparable maps to conventional 2D methods (Fig. 3). AHA 16-segment left-ventricle analysis was performed on T1/T2 maps: mean segment values and coefficients of variation (CoV) were measured (Fig. 4). Mean left ventricular T1 values were 1071±25 ms, 1171±26 ms and 1121±36 ms for 2D MOLLI, SASHA and 3D cMRF, respectively. T2 mean values were 52.6±1.4 ms and 46.1±2.7 ms for 2D T2-GraSE and 3D cMRF, respectively. Corresponding CoV T1 values were 8.1±2.3 %, 12.2±3.3 % and 5.4±0.6 %, ms for 2D MOLLI, SASHA and 3D cMRF, respectively; CoV T2 values 10.6±1.5 % and 10.9±1.3 % for T2-GraSE and 3D cMRF, respectively. 3D cMRF produced co-registered, whole-heart T1 and T2 mapping with good definition of the myocardium and papillary muscles over the entire volume (Fig. 5).CONCLUSION:
3D free-breathing cardiac MRF was developed for simultaneous, co-registered and whole-heart T1/T2 mapping in a predictable scan time of ~7 min. 3D cMRF was in general agreement with conventional methods for phantom and in vivo experiments. Future work will consider more complex motion models for cMRF and further validation in patients.Acknowledgements
ACKNOWLEGDMENTS: This work was supported by EPSRC (EP/P001009, EP/P032311/1) and Wellcome EPSRC Centre for Medical Engineering (NS/ A000049/1).References
1. Patel AR, Kramer CM. Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. JACC Cardiovasc. Imaging 2017;10:1180–1193 doi: 10.1016/j.jcmg.2017.08.005
2. Hamilton JI, Jiang Y, Chen Y, et al. MR fingerprinting for rapid quantification of myocardial T 1 , T 2 , and proton spin density. MRM 2017;77:1446–1458.
3. Cheng JY, Alley MT, Cunningham CH, Vasanawala SS, Pauly JM, Lustig M. Nonrigid motion correction in 3D using autofocusing with localized linear translations. MRM 2012;68:1785–1797.
4. Assländer J, Cloos MA, Knoll F, Sodickson DK, Hennig J, Lattanzi R. Low Rank Alternating Direction Method of Multipliers Reconstruction for MR Fingerprinting. MRM 2017; 79:83-96. doi:10.1002/mrm.26639.
5. Bustin A, Cruz G, Jaubert O, Lopez K, Botnar RM, Prieto C. High - dimensionality undersampled patch - based reconstruction ( HD - PROST ) for accelerated multi - contrast MRI. Magn. Reson. Med. 2019:3705–3719 doi: 10.1002/mrm.27694.
Figures