1078
Association of Brain Biomechanics and Vascular Dynamics using 4D Flow, MRE and DENSE MRI1Department of Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin - Madison, Madison, WI, United States, 3Department of Medicine, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
The coupling of brain biomechanics and hemodynamics is complex as it includes arterial pressure pulsations, venous and CSF flow, and tissue compliance. Experimental evidence has demonstrated alterations of each the multiple compartments in disease; however, the relationships and coupling between brain biomechanics (e.g. strain and stiffness) and vascular flow dynamics is not well characterized. This study investigates the relationships between brain blood flow, stiffness, and strain using a multi-scale brain imaging platform that includes 4D flow, MRE, and DENSE MRI. Results suggest strong correlations between blood flow, strain, and stiffness and age-related changes in these parameters.
Introduction:
The interplay between cranial arterial pressure pulsations, CSF and venous flow, and tissue compliance is complex and is implicated in mechanisms driving glympathic flow, removing interstitial fluid and soluble metabolites from the brain.1,2 Pathologies such as dementia can manifest with altered biomechanics in different cranial compartments (e.g. tissues, vessel walls) and at different scales (e.g. small displacements in tissue, fast flow in arteries).3,4 Further, normal aging is associated with decreased brain stiffness but increased vascular stiffness.5,6 Yet, the relationships between vascular flow-induced brain strain and tissue stiffness are poorly understood, severely limiting its potential use for the detection of early disease and disease monitoring. Noninvasive quantitative assessment of vascular flow, cardiac induced tissue strain, and tissue stiffness in the brain is feasible with MRI.7,8,5 However, studies to date have not acquired these metrics in the same study population or attempted to evaluate their associations. In this work, we explore the correlation between macroscopic blood flow, cardiac induced brain tissue strain, brain stiffness and age using a multi-scale brain imaging MRI protocol that includes radial 4D flow, 2D single-shot DENSE MRI, and EPI MR Elastography (MRE).Methods:
Subjects: A total of 32 healthy volunteers participated in this study (mean age =51±20yrs, range =[23, 82]yrs, 13 females). MRI: Volumetric, time-resolved phase contrast (PC) MRI data with 4-point referenced encoding were acquired in all subjects on a 3.0T system (Signa Premier, GE Healthcare) using a 48-channel head coil (GE Healthcare), with a 3D radially-undersampled sequence 9, with the following imaging parameters Venc=80cm/s, imaging volume =22x22x10cm3, TR/TE=7.7/2.5ms, 0.7mm acquired isotropic resolution, and scan time=5.6min. Flow encoded images were reconstructed to 20 cardiac phases using retrospective ECG gating and view sharing.10 Prospectively gated 2D single-shot spiral DENSE images were also acquired in all 32 subjects using a ramped flip angle approach 8, Denc=0.175mm, 1 slice, FOV=24x24cm2, dz=1cm, 1.9x1.9mm2 in-plane resolution, 10 cardiac time frames, TR/TE~100/2.1ms and scan time~40sec. Finally, multi-slice EPI MRE data were collected in 13 of the 32 subjects (mean age=29±6yrs, range=[23,43]yrs, 3 females) by transmitting shear waves with 60 Hz vibration frequency to the brain using a pillow-like passive driver with eight phase offsets sampled over one period of 60 Hz motion. Additional scan parameters included imaging volume = 24x24x14cm3, TR/TE = 3200/70ms, 3.3x3.3x3mm3 resolution, and scan time~5min. Flow, Strain and Stiffness analysis: Phase data were unwrapped using a Laplacian approach prior to generation of velocity and displacement images.11 Blood flow rates from the left and right petrous internal carotid arteries were extracted from the velocity data in MATLAB (Mathworks, Natick, MA).12 Tissue displacement was measured from DENSE images from ROIs placed in brain tissue proximal to the lateral ventricles. Strain maps were derived by calculating the divergence of the displacement field and quantified using the same ROIs.8 Stiffness maps from MRE data were generated using a 3D direct inversion algorithm and stiffness was quantified using the same ROIs.13,14 Coefficient of determination were derived from linear regression modeling. Group differences were assessed using Student’s t-test (P<0.05).Results:
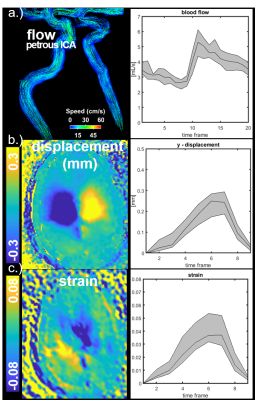
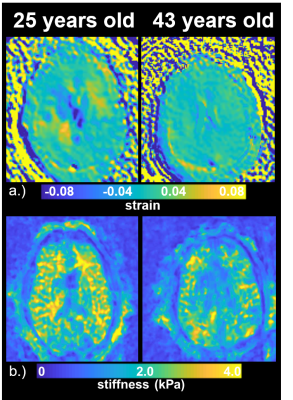
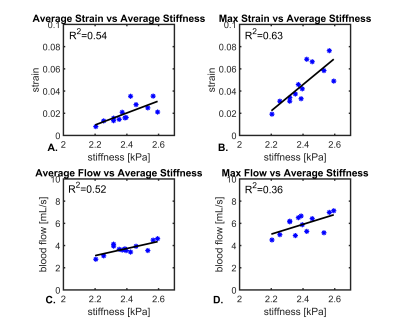
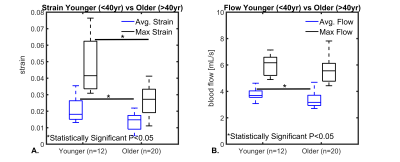
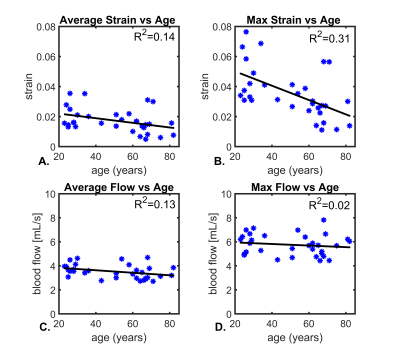
Figure 1 shows example images from a healthy volunteer including velocity fields derived from 4D flow, displacement fields from 2D DENSE, and strain maps from the displacement field divergence. In addition, mean values for a group of young healthy volunteers (<40yrs, n=12) are shown. Color-coded strain and stiffness maps at the level of the lateral ventricles are shown for two healthy volunteers (Figure 2). Higher strain and stiffness are appreciable in the younger volunteer (left column) (25 vs 43yrs). Figure 3 shows linear regression models assessing the correlations between strain, flow, and stiffness. Results showed moderate-to-strong correlation between stiffness and average blood flow (R2=0.52) and max tissue strain (R2=0.63). Boxplots in Figure 4 show significantly higher average and max strain in younger subjects when compared to older subjects (P=0.033, P<0.001). Furthermore, average blood flow was also significantly higher in younger subjects (P=0.028). Finally, Figure 5 shows linear regression analysis correlating strain, flow, and age. Both strain and flow decreased with age; however, both were weakly correlated, and the highest correlation was between max strain and age (R2=0.31).Discussion and Conclusions:
This study measured and analyzed cranial blood flow, displacement, strain, and stiffness in a healthy cohort. Through the measurements of single-shot spiral 2D DENSE and multi-slice MRE displacement fields, brain tissue strain and stiffness were correlated (R2=0.63). Macroscopic blood flow was also moderately correlated with tissue stiffness (R2=0.52). Overall, age effect analysis showed a significant decrease (P<0.05) in strain and blood flow in older adults when compared to a younger group. These findings agreed well with studies that observed decreased overall brain stiffness and blood flow in older adults.5,6 Further, decreased cerebral blood flow and brain elasticity have been observed in animal models that used drug-induced hypotension, suggesting a need for mechanical stimuli driven by the vascular system to sustain tissue structural integrity.15 Age-dependent modifications of mechanical properties at the cellular and functional level also likely lead to changes in viscoelastic properties of the brain.16 Future studies will aim to elucidate the relationship between vascular and tissue structure to intracranial biomechanic forces. Longitudinal data comparing these parameters across age and pathology could offer valuable insights into underlying disease pathogenesis.Acknowledgements
We gratefully acknowledge research support from GE Healthcare, and funding support from NIH grants R01NS066982, R01HL136965, P50-AG033514, and R01AG021155.References
1. Arbel-Ornath M, Hudry E, Eikermann-Haerter K, et al. Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer's disease mouse models. Acta Neuropathol. 2013 Sep;126(3):353-64. doi: 10.1007/s00401-013-1145-2.
2. Mestre H, Kostrikov S, Mehta RI, et al. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond). 2017 Aug 10;131(17):2257-2274. doi: 10.1042/CS20160381.
3. ElSheikh M, Arani A, Perry A, et al. MR Elastography Demonstrates Unique Regional Brain Stiffness Patterns in Dementias. AJR Am J Roentgenol. 2017 Aug;209(2):403-408.
4. Berman SE, Rivera-Rivera LA, Clark LR, et al. Intracranial Arterial 4D-Flow is Associated with Metrics of Brain Health and Alzheimer's Disease. Alzheimers Dement (Amst). 2015 Dec 1;1(4):420-428.
5. Takamura T, Motosugi U, Sasaki Y, et al. Influence of Age on Global and Regional Brain Stiffness in Young and Middle-Aged Adults. J Magn Reson Imaging. 2019 Aug 1. doi: 10.1002/jmri.26881. [Epub ahead of print]
6. Rivera-Rivera LA, Johnson SC, Illingworth C, et al. Intracranial Pulse Wave Velocity in Alzheimer’s Disease using Flow Encode Split and Low Rank Reconstructed 4D Flow MRI, 27th Annual ISMRM Meeting, Montreal, Canada, p. 622
7. Rivera-Rivera LA, Turski P, Johnson KM, et al. 4D flow MRI for intracranial hemodynamics assessment in Alzheimer's disease. J Cereb Blood Flow Metab. 2016 Oct;36(10):1718-1730.
8. Adams AL, Kuijf HJ, Viergever MA, et al. Quantifying cardiac-induced brain tissue expansion using DENSE. NMR Biomed. 2019 Feb;32(2):e4050. doi: 10.1002/nbm.4050.
9. Johnson KM, Lum DP, Turski PA, et al. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med. 2008 Dec;60(6):1329-36.
10. Liu J, Redmond MJ, Brodsky EK, et al. Generation and visualization of four-dimensional MR angiography data using an undersampled 3-D projection trajectory. IEEE Trans Med Imaging. 2006 Feb;25(2):148-57.
11. Loecher M, Schrauben E, Johnson KM, et al. Phase unwrapping in 4D MR flow with a 4D single-step laplacian algorithm. J Magn Reson Imaging. 2016 Apr;43(4):833-42.
12. Schrauben E, Wåhlin A, Ambarki K, et al. Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries. J Magn Reson Imaging. 2015 Nov;42(5):1458-64.
13. Oliphant TE, Manduca A, Ehman Rl, et al. Complex-valued stiffness reconstruction for magnetic resonance elastography by algebraic inversion of the differential equation, Magn. Reson. Med. 45 (2001) 299–310.
14.Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity, Med. Image Anal. 5 (2001) 237–254.
15. Chatelin S, Humbert-Claude M, Garteiser P, et al. Cannabinoid receptoractivation in the juvenile rat brain results in rapid biomechanical alterations: Neurovascular mechanism as a putative confounding factor. Journal of Cerebral Blood Flow and Metabolism. 2016;36:954–964.
16. Lu YB, Iandiev I, Hollborn M, et al. Reactive glial cells: Increased stiffness correlates with increased intermediate filament expression. FASEB J 2011; 25: 624– 631.
Figures