1073
High resolution 4D vessel selective angiography in under 5 minutes using a constrained reconstruction1Wellcome Centre for Integrative Neuroimaging, NDCN, University of Oxford, Oxford, United Kingdom
Synopsis
Arterial spin labeling methods can be used to produce vessel selective angiograms. However, to do this in 3D or 4D is extremely time consuming as many encodings of high spatial (and temporal) resolution images are needed. We propose an optimized acquisition and reconstruction method to create high quality angiogams is five minutes or less. For the acquisition protocol we explore different sampling patterns across encoded images, and for the reconstruction method different ways of constraining the signal temporally.
Introduction
Vessel-encoded arterial spin labelling (VE-ASL1angiography is an MR method for studying artery-specific dynamic blood flow in the brain, with clinical applications in the diagnosis and management of cerebrovascular diseases such as stenosis, arteriovenous malformations, and stroke.VE-ASL angiography is performed like pseudo-continuous ASL with additional transverse gradients to allow for some vessels to be tagged and others to be controlled. This way, multiple ‘encoded’ images can be acquired, allowing for blood originating in different arteries to be decoded. However, fully sampled 3D time-resolved (4D) VE-ASL angiography is extremely time-consuming, requiring hours of scan time.
Methods like parallel imaging (PI2,3) or compressed sensing (CS4,5) allow for undersampled reconstruction by relying on external information (i.e., coil sensitivity or image compressibility). Here, we present a constrained reconstruction framework for VE-ASL that can bring acquisition durations down to clinically feasible times (10, 5, or 1 min). We also explore how to best regularize the temporal domain of the data and variations of the acquisition protocol to be more compatible with a CS-type reconstruction.
Methods
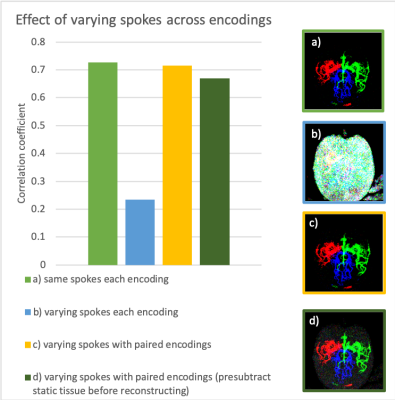
Using a 3D golden angle6,7radial trajectory, we explored the effect of varying spokes across vessel encodings, to spread the aliased signal across space and vessel components. However, to limit leakage of strong static tissue signal into the vessel images, a hybrid encoding scheme to remove static tissue signal was used (Fig. 1a). This was achieved by acquiring pairs of opposed vessel encodings with matched trajectories such that their subtraction fully removes the static tissue. This increases the number of vessel encodings by a factor of two, so the number of spokes acquired for each encoding were halved for matched scan time.The temporal evolution of the signal follows the smooth dynamic model described by Okell et al.8. We included this as a constraint in the reconstruction as either a L1-constraint with a temporal model-based sparsifying transform, or a simple L2-temporal smoothness constraint.
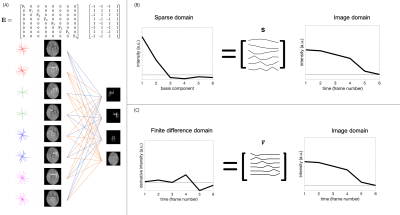
The reconstruction was performed using FISTA9 to minimize the following cost function:
$$cost = \frac{1}{2} |\mathbf{Ex}-\mathbf{d}|^2_2+\lambda_1 | \mathbf{Sx}|_1 + \frac{1}{2} \lambda_2 |\mathbf{\nabla x}|_2^2$$
Where $$$\mathbf{E}$$$ consists of the vessel encoding, coil sensitivity profiles, and a Fourier sampling operator. Here, $$$\mathbf{x}$$$ is the estimated image (one vector containing all spatial, temporal, and vessel components concatenated), $$$\mathbf{d}$$$ is the acquired data, $$$\mathbf{\nabla}$$$ is the temporal finite difference operator (Fig. 1c), and $$$\lambda_1$$$ and $$$\lambda_2$$$ are weights on the sparsity and temporal smoothness terms respectively. When the temporal model was used, $$$\mathbf{S}$$$ was set to the model-derived temporal basis (Fig. 1b) and $$$\lambda_2$$$ was set to zero. When no temporal model was used, $$$\mathbf{S}$$$ was set to the identity transform.
Simulations were performed on a 128x128x86 numerical phantom with 6 temporal frames based on data acquired for a previous study10 (resolution: 1.6 mm2isotropic, temporal resolution: 210 ms).
Initial feasibility data were also acquired in-vivo from one healthy volunteer. Resolution: 1.1 mm2isotropic, temporal resolution: 210 ms. Total scan time 10 min (acceleration factor ~39). The labeling duration was 500ms. Image quality was quantified by calculating the correlation coefficient between all pixels in a vessel mask of the reconstruction and the ground truth.
Results
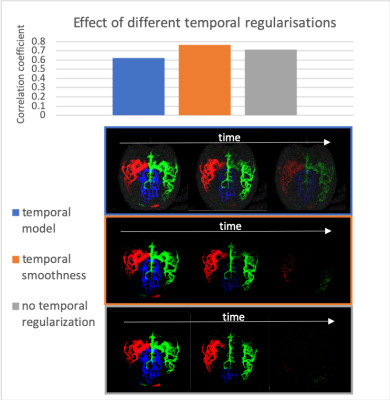
Figure 2 shows the effect of different sampling schemes on the simulated data. Using the same spokes for each encoding and the paired encodings with varying spokes scheme, as proposed above, had the best, and approximately equal, correlation coefficients. As expected, the freely varying spokes approach did the worst due to strong static tissue aliasing. Pre-subtracting the static tissue resulted in visibly higher noise than reconstructing using all the acquired data.Regularizing the temporal dimension resulted in higher correlation coefficients when using the smoothness constraint, but the sparse temporal model worsened the result (Fig. 3). The temporal model resulted in residual signal in the later frames that drove down the correlation coefficient.
The in-vivo data was reconstructed with 4 different combinations of acquisition method and temporal regularization (Fig. 4). Here, the paired encodings with varying spokes method with temporal smoothing produced the visibly highest quality images, with reduced background noise and higher vessel contrast.
Figure 5 shows the in-vivo varying spokes + temporal smoothness data in more detail and how the reconstruction behaves as the data gets more and more undersampled, with considerable retention of image quality, even in only 5 minutes of data.
Discussion
We have produced high-quality highly accelerated 4D angiograms in-vivo and explored different sampling and temporal regularization methods. The varying spokes approach produced higher quality results in-vivo than in simulations, relative to the same-spokes encoding. This discrepancy could be explained by the differences in image dimensions leading to different optima for the regularization factors. Further investigation into the interactions between encoding and sampling in a joint decoding and reconstruction framework are needed to fully explain these results.Temporally, we found that the simpler smoothness constraint worked better at regularizing the reconstruction and maintaining the temporal fidelity than using a kinetic model. This could be explained by the low number of frames acquired and therefore low compressibility of the temporal signal.
Conclusion
By joint consideration of both the acquisition and spatio-temporal reconstruction, high-quality 4D VE-ASL angiograms can be acquired in 5 minutes or less.Acknowledgements
The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z). MC (RF201617\16\23) and TO (RF/132) are supported by the Royal Academy of Engineering. SS was supported by funding from the Engineering and Physical Sciences Research Council (EPSRC) and Medical Research Council (MRC) (EP/L016052/1)References
1. Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magn. Reson. Med. 2007;58:1086–1091 doi: 10.1002/mrm.21293.
2. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity Encoding for Fast MRI. 1999:11.
3. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic Resonance in Medicine 2002;47:1202–1210 doi: 10.1002/mrm.10171.
4. Lustig M, Donoho DL, Santos JM, Pauly JM. Compressed Sensing MRI. IEEE Signal Processing Magazine 2008;25:72–82 doi: 10.1109/MSP.2007.914728.
5. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007;58:1182–1195 doi: 10.1002/mrm.21391.
6. Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An Optimal Radial Profile Order Based on the Golden Ratio for Time-Resolved MRI. IEEE Trans. Med. Imaging 2007;26:68–76 doi: 10.1109/TMI.2006.885337.
7. Chan RW, Ramsay EA, Cunningham CH, Plewes DB. Temporal stability of adaptive 3D radial MRI using multidimensional golden means. Magn. Reson. Med. 2009;61:354–363 doi: 10.1002/mrm.21837.
8. Okell TW, Chappell MA, Schulz UG, Jezzard P. A kinetic model for vessel-encoded dynamic angiography with arterial spin labeling. Magn Reson Med 2012;68:969–979 doi: 10.1002/mrm.23311.
9. Beck A, Teboulle M. A Fast Iterative Shrinkage-Thresholding Algorithm for Linear Inverse Problems. SIAM J. Imaging Sci. 2009;2:183–202 doi: 10.1137/080716542.
10. Schauman SS, Chiew M, Okell TW. Highly Accelerated Dynamic 2D and 3D Vessel-Encoded Arterial Spin Labelling Angiography. In: Annual Meeting of ISMRM 2019. ; 2019.
Figures