1069
Effects from inhalation of hypoxic air and carbon monoxide exposure on human cerebral perfusion, oxygen consumption and lactate production1Functional Imaging Unit, Department of Clinical Physiology, Nuclear Medicine and PET, Copenhagen University Hospital Rigshospitalet, Glostrup, Denmark, 2Danish Headache Center, Department of Neurology., Copenhagen University Hospital Rigshospitalet, Glostrup, Denmark, 3Neurobiology Research Unit, Department of Neurology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark
Synopsis
In present study we demonstrate that in healthy humans the cerebral lactate concentration increases during inhalation of hypoxic air but not after exposure to carbon monoxide. This suggests a regulatory mechanism of cerebral glycolytic activity possibly mediated by sensing of arterial oxygen pressure and that the lactate production is not solely a result of hindered oxidative metabolism, at least during non-threatening hypoxic exposure. Phase-contrast mapping and susceptibility-based oximetry were used to acquire global cerebral blood flow and oxygen consumption and MR-spectroscopy was used to measure the lactate concentration in the occipital lope in a total of 51 healthy humans.
Introduction
Brain physiology and metabolism is tightly regulated to ensure normal neuronal function. Hypoxic exposure causes increased cerebral perfusion to maintain adequate oxygen delivery to the brain. However, inhalation of hypoxic air also increases the cerebral lactate production even though the brain oxygen consumption is unaffected, suggesting a metabolic adaption1,2. In present study we investigate the mechanism of this lactate production from hypoxic exposure and explore for possible correlation with brain perfusion, oxygen availability and oxygen consumption. This was done by examining a group of healthy humans inhaling hypoxic air while lying in the MRI-scanner and a group that immediately before scanning was exposed to air with carbon monoxide (CO).Inhalation of hypoxic air causes desaturation of arterial oxyhemoglobin (SaO2) and decreases the arterial oxygen partial pressure (PaO2), whereas inhalation of CO only decreases SaO2, by CO-molecules binding to the hemoglobin, but PaO2 is maintained at normal pressure3,4. This enables us to distinguish between the effects from general lowered oxygen concentration in the arterial blood from that of arterial oxygen partial pressure.
Global mean cerebral blood flow (gCBF) and global mean cerebral metabolic rate of oxygen (gCMRO2) were acquired noninvasively using phase-contrast MRI technique and lactate concentration were acquired using MR spectroscopy.
Methods
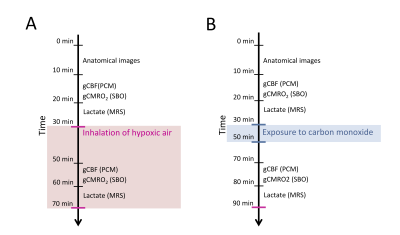
51 subjects participated in the study. 30 subjects inhaled hypoxic air, and 21 were exposed to air with CO. All MRI scans were performed on a Philips 3T Achieva dSTREAM MRI scanner (Philips Medical Systems, Best, The Netherlands) using a 32-channel phase array head coil. The acquisition of parameters was covered in one MRI session. Lactate concentration, gCBF and gCMRO2 were acquired at resting baseline and measurements were repeated during inhalation of hypoxic air or after CO exposure. The approximate timing of the acquisition of the parameters is demonstrated in figure 1.Phase-contrast mapping (PCM) were used to acquire the blood flow in the feeding cerebral arteries by acquiring blood velocity maps using a turbo field echo sequence (1 slice, voxel size=0.75×0.75×8 mm3; velocity encoding=100 cm/s, without cardiac gating).
Susceptibility-based oximetry (SBO) was used to measure the venous oxygen saturation in the blood leaving the brain in the sagittal sinus5. SBO utilizes that the different magnetic susceptibility between deoxyhemoglobin and oxyhemoglobin can be related to oxygen saturation. Susceptibility-weighted images were obtained by a dual-echo gradient-echo sequence and subtracting the phase-images from each echo (voxel size=0.7×0.7×8 mm3; TE1=10.89 ms; TE2=24.16 ms; velocity encoding=100 cm/s). The sequence was interleaved with a velocity-encoding scheme to also acquire the blood flow in the sagittal sinus. By using the Fick’s principle gCMRO2 could then be calculated. Cerebral lactate concentration was measured in the occipital lobe by MR-spectroscopy using a water-suppressed point-resolved spectroscopy (PRESS) pulse sequence (TE=36 ms; voxel size=30×35×30 mm3). Anatomical images were obtained by a 3D T1-weighted turbo field echo sequence (voxel size=1.1×1.1×1.1 mm3; TE=2.78 ms; TR=6.9 ms; flip angle=9°).
The effects from exposure to hypoxic air or CO on gCBF, gCMRO2 and lactate concentration were assessed by a linear mixed model.
Results
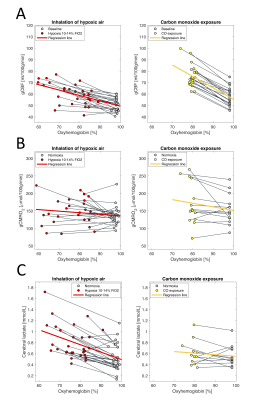
The arterial oxyhemoglobin saturation was on average 75.5±8.0% during inhalation of hypoxic air and 79.2±1.9% after carbon monoxide exposure at the time of acquisition of the MRI parameters.Cerebral lactate concentration, gCBF and gCMRO2 at baseline and during exposure to hypoxic air or after exposure to CO are shown in figure 2. Inhalation of hypoxic air caused an increase in gCBF of 20% from 50.7±5.5 ml/100g/min to 60.5±9.5 ml/100g/min (p<10-6). The gCMRO2 was unaffected from 136.3±25.49 mmol/100g/min at baseline to 147.8±38.2 mmol1gmin (p=0.13) during inhalation of hypoxic air. Lactate concentration increased with 71% from 0.54±0.24 mmol/l to 0.81±0.30 mmol/l (p<10-6).
Exposure to CO increased CBF with 33.0% from 57.3±7.9 ml/100g/min to 75.9±9.7 ml/100g/min (p<10-6). The increase was significantly higher than that from inhalation of hypoxic air (p=0.0024). The gCMRO2 was unaffected from CO exposure (p=0.071), and with no difference compared to inhalation of hypoxic air (p=0.30). Exposure to CO did not increase lactate concentration from 0.56±0.19 mmol/l at baseline to 0.61±0.22 mmol/l after exposure to CO (p=0.26). The lactate increase from the hypoxic challenge was significantly different than from CO exposure (p<10-3).
Discussion and conclusion
In present study we found that CO exposure does not affect the cerebral lactate concentration, whereas inhalation of hypoxic air robustly increases the lactate concentration. The higher lactate production occurs even though the cerebral oxygen consumption was unaffected. This suggests that glycolytic activity and lactate production in the human brain is dynamically regulated, possible by a direct sensing mechanism of arterial oxygen partial pressure, and hypoxia-stimulated cerebral lactate production is not solely a result of inadequate oxygen availability and hindered oxygen metabolism. It remains to be established whether CO2 alteration is involved in the lactate change and future studies should address the influence of CO2 on the lactate flux.Acknowledgements
No acknowledgement found.References
1. Vestergaard MB, Lindberg U, Aachmann-Andersen NJ, Lisbjerg K, Christensen SJ, Law I et al. Acute hypoxia increases the cerebral metabolic rate – a magnetic resonance imaging study. J Cereb Blood Flow Metab 2016; 36: 1046–1058.
2. Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 1948; 27: 484–492.
3. Arngrim N, Schytz HW, Britze J, Vestergaard MB, Sander M, Olsen KS et al. Carbon monoxide inhalation induces headache in a human headache model. Cephalalgia 2018; 38: 697–706.
4. Paulson OB, Parving HH, Olesen J, Skinhoj E. Influence of carbon monoxide and of hemodilution on cerebral blood flow and blood gases in man. J Appl Physiol 1973; 35: 111–116.
5. Rodgers ZB, Leinwand SE, Keenan BT, Kini LG, Schwab RJ, Wehrli FW. Cerebral metabolic rate of oxygen in obstructive sleep apnea at rest and in response to breath-hold challenge. J Cereb Blood Flow Metab 2016; 36: 755–67.
Figures