1063
Investigation of black blood MRI signal enhancement in a patient-specific aneurysm model.
Mariya Stanislavovna Pravdivtseva1, Carson Hoffman2, Leonardo A. Rivera-Rivera2, Rafael Medero2, Lindsay Bodart2, Alejandro Roldan-Alzate2, Michael A. Speidel2, Charles M. Strother2, Kevin M. Johnson2, Oliver Wieben2, Olav Jansen3, Naomi Larsen3, Philipp Berg4, Eva Peschke1, and Jan-Bernd Hövener1
1Neuroradiology and Radiology, Section Biomedical Imaging, Molecular Imaging North Competence Center (MOIN CC), Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein (UKSH), Kiel University, Kiel, Germany, 2Department of Medical Physics, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA, Madison, WI, United States, 3Neuroradiology and Radiology, Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein (UKSH), Kiel, Germany, 4Research Campus STIMULATE, University of Magdeburg, Magdeburg, Germany, Magderburg, Germany
1Neuroradiology and Radiology, Section Biomedical Imaging, Molecular Imaging North Competence Center (MOIN CC), Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein (UKSH), Kiel University, Kiel, Germany, 2Department of Medical Physics, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA, Madison, WI, United States, 3Neuroradiology and Radiology, Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein (UKSH), Kiel, Germany, 4Research Campus STIMULATE, University of Magdeburg, Magdeburg, Germany, Magderburg, Germany
Synopsis
Intracranial aneurysm is a life-threatening disease. Vessel wall enhancement may be used as a marker to identify an aneurysm with a high risk of rupture. Accumulation of contrast agent in the vessel wall and slow or turbulent flow can contribute to the formation of vessel wall enhancement. In the current study enhanced signal on black blood MRI was observed in printed model of an intracranial aneurysm with and without Gd administration. The found signal was associated with the slow flow in the aneurysm. Additionally, the impact of spatial resolution, flow rate, MSDE preparation and contrast concentration was considered.
Introduction:
Intracranial aneurysms are a common pathology and reliable assessment of rupture risk of life-threatening intracranial haemorrhage remains a clinical challenge. Recent retrospective studies have found that vessel wall enhancement (VWE) may allow identification of aneurysms with a high risk of rupture1. The reason for VWE is yet unknown; hypotheses include accumulation of contrast agent in the vessel wall and slow, unstable or turbulent flow. The latter has been described to cause pseudo-enhancement on 3D T1-weighted turbo spin echo (TSE) black blood MRI (BB MRI) in vivo2, possibly impeding precise assessment of true wall enhancement. Likewise, a similar effect was observed in vitro in a plastic model connected to a flow pump3, even though this model had impermeable walls and hence contrast cannot possibly accumulate.The aim of this study was to investigate intra-aneurysmal hyperintensities in a 3D printed patient-specific aneurysm model to gain a better understanding of the nature of BB MRI wall enhancement signal.
Methods:
Model: A patient with BB MRI enhancement in a large saccular intracranial aneurysm after the administration of 0.1 mmol/kg gadoterate meglumine (Dotarem, Guerbet, Villepinte, France) was identified. A model of the aneurysm was constructed based on in vivo 3D rotational angiographic (3D RA) data and printed using stereolithography (Form 2, Formlabs). The model was placed into an agarose gel and connected to a pulsatile pump set to an averaged flow of 250 or 300 ml/min (tap water, PD-1100, BDC laboratories).MRI measurements were performed on a 3 T MR scanner equipped with a 48 channel head-coil (Signa Premier, GE Healthcare Waukesha, WI, USA). A T1-weighted variable refocusing flip angle 3D TSE sequence (TE/TR = 27/1000 ms; matrix 320ꞏ320; imaging volume 220x220x102 mm3; 3 acquired voxel sizes: 0.9x0.9x0.8; 0.7x0.7x0.8; 0.5x0.5x0.8 mm3) before and after administration of gadolinium-based contrast agent (Magnevist, 0.3 or 0.6 ml / L concentration in pump fluid) with and without motion-sensitized driven equilibrium (MSDE) preparation4.
Time-resolved phase-contrast (PC) MRI with 3D velocity encoding was acquired with isotropic-voxel radial projection imaging (PCVIPR)5 (TE/TR 2.8/12 ms; flip angle 8°; field of view 220x220x120 mm3; acquired voxel size: 0.5x0.5x0.5 mm3; velocity encoding 50 cm/s). Reconstruction was performed offline with customized software.
Image-based blood flow simulations were carried out using STAR-CCM+ 13.04 (Siemens PLM Software Inc., Plano, TX, USA). Identical flow conditions (250 ml/min) were set up compared to the experiment, however, a considerably higher spatial resolution of 0.15 mm was chosen (leading to approx. 4.2 million polyhedral and prism elements).
Evaluation: On BB MR images, the signal was measured in three regions of interest (ROI): aneurysm lumen, aneurysm wall, and agarose gel. To compensate for variability in between scans, the mean signal of the aneurysm lumen and wall was normalized by the agarose signal for further analysis. Standard deviations were calculated over the ROIs. The Pearson correlation coefficient was calculated between the PC MRI and BB VWE data pixelwise over the aneurysm (ROI B and C, Figure 1).
Results:
The in vitro setup was successfully implemented and a hyperintense signal BB MRI was found in the lumen and adjacent to the aneurysm walls, similar to the findings in vivo (Figure 2). The signal in the lumen and wall was stronger after Gd administration. Both Gd concentrations showed the same result (Figure 3). The signal was reduced when higher flow, higher spatial resolution or MSDE was used (Figure 4).In the same experiment, 4D flow MRI was successfully measured. Interestingly, the flow was found to negatively correlate with the BB MRI signal (ROI B, Figure 5): areas of decreased flow exhibited increased BB MRI signal.
The hemodynamic simulation reveals slow flow areas, especially in regions, where the highest signal enhancement is visible.
Discussion:
Our results indicate that the BB MRI signal in the aneurysm lumen is associated with slow flow inside the aneurysm. This hypothesis is supported by the finding that the hyperintense signal correlates negatively with the velocity. These results are in line with similar findings reported previously3. In addition, VWE was found to decrease at higher spatial resolution. This is in line with the expected increased flow sensitivity associated with the increased spatial encoding and crushing gradients. While a higher resolution reduces the enhancement, it prolongs the acquisition time significantly. MSDE was found to reduce the enhancement further, which is in accordance with previously published data6. MSDE does not prolong the sequence much, which makes it a more practical choice for suppressing VWE in clinical routine than imaging with higher resolution.To conclude about observed wall enhancement, the contribution of wall roughness has to be investigated. 3D printed model is different from a vessel in vivo. Still, a very similar BB MRI signal was found. Deliberately increasing or decreasing the permeability of the walls may elucidate the origin of this effect further (e.g. by sealing the surface with coating or adding permeable microstructure).
Conclusion:
Enhanced BB MRI signal was observed in an in vitro model of an intracranial aneurysm with and without Gd administration. A higher flow rate, higher spatial resolution, and MSDE preparation reduced this effect. Printed models may help to optimize sequences and to find out, where the inflammation associated vessel wall enhancement actually originates from.Acknowledgements
We are grateful for the financial and intellectual support by the Research Training Group “Materials4Brain” (GRK2154; P2, P10).References
1. Edjlali, M. et al. Does Aneurysmal Wall Enhancement on Vessel Wall MRI Help to Distinguish Stable From Unstable Intracranial Aneurysms? Stroke 45, 3704–3706 (2014). 2. Cornelissen, B. M. W. et al. Vessel wall enhancement of intracranial aneurysms: fact or artifact? Neurosurg. Focus 47, E18 (2019). 3. Cornelissen, B. M. W. et al. Insufficient slow-flow suppression mimicking aneurysm wall enhancement in magnetic resonance vessel wall imaging: a phantom study. Neurosurg. Focus 47, E19 (2019). 4. Wang, J., Yarnykh, V. L. & Yuan, C. Enhanced Image Quality in Black-Blood MRI by Using the Improved Motion-Sensitized Driven-Equilibrium (iMSDE) Sequence. J. Magn. Reson. Imaging JMRI 31, 1256–1263 (2010). 5. Gu, T. et al. PC VIPR: A High-Speed 3D Phase-Contrast Method for Flow Quantification and High-Resolution Angiography. Am. J. Neuroradiol. 26, 743–749 (2005). 6. Kalsoum, E. et al. Blood Flow Mimicking Aneurysmal Wall Enhancement: A Diagnostic Pitfall of Vessel Wall MRI Using the Postcontrast 3D Turbo Spin-Echo MR Imaging Sequence. Am. J. Neuroradiol. 39, 1065–1067 (2018).Figures
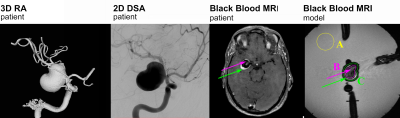
Figure
1. 3D RA, 2D
digital subtraction angiography (DSA) and BB MRI of the patient chosen for this
study. 3D
RA was used to generate the model in silico. BB MRI of the patient and model showed signal enhancement in the lumen (pink arrows) and
walls of the aneurysm (green arrows). The regions used
for quantifying the effect in the lumen and wall are indicated (A, B, C).
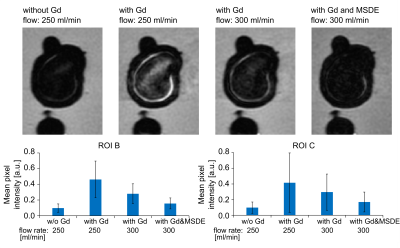
Figure
2. In vitro BB MRI before and after Gd administration for
different flow rates and with or without MSDE preparation.
The signal in the lumen was stronger after Gd administration and reduced when a higher flow or MSDE was used. The presented data were acquired with spatial resolution 0.7x0.7x0.8 mm3
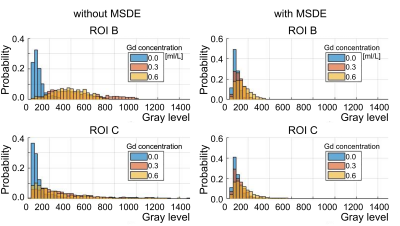
Figure 3. The probability histograms
of pixel intensity in the aneurysm lumen (ROI B, top) and wall (ROI C, bottom) without
MSDE (left) and with MSDE (right). The signal was stronger
after Gd administration. However, the increased rate of Gd didn’t cause an increase in
BB MRI signal. MSDE minimized the differences between pixel intensity distribution before and after Gd
administration.
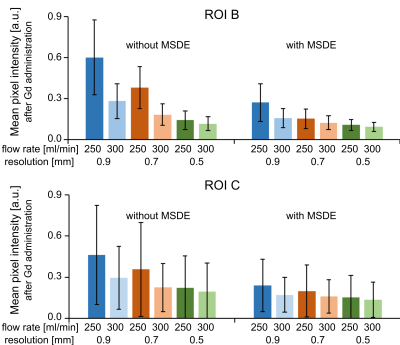
Figure 4. Normalized BB
MRI signal in the aneurysm lumen. The signal is decreasing with increasing
spatial resolution, flow rate. The MSDE preparation decreased the signal for
all resolutions and flow rates further.
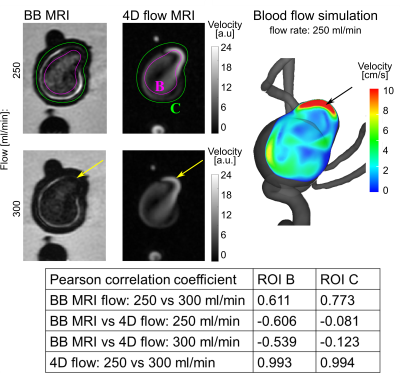
Figure 5. Magnitude BB MRI
(left) and 4D flow MRI (middle) of the aneurysm model for different flow rates. Blood flow simulation (right) was performed for one flow rate. Note
that high 4D flow MRI signal (which is averaged over time) and velocity obtained by simulation corresponds to some of the areas with low signal on the BB
MRI images (e.g. yellow and black arrows).