1062
Automated Morphology Analysis of Intracranial and Extracranial Vessel Wall Using Convolutional Neural Network1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Synopsis
Intracranial and extracranial atherosclerotic disease are major causes of ischemic stroke. Manual analyses of intracranial and extracranial artery vessel wall are time consuming and experience dependent. The purpose of this study was to develop an automated method to analyze 3D intra- and extracranial arterial vessel wall images, including vessel centerline tracking, vessel straightened reformation, vessel wall segmentation based on CNN, and morphological quantification. In conclusion, the proposed method facilitates the large-scale quantitative analysis of vessel wall, and is promising in promoting the clinical applications of MR vessel wall imaging.
Introduction
Atherosclerotic plaque rupture of intracranial and extracranial arteries is major cause of ischemic stroke [1, 2]. In recent years, several high-resolution isotropic 3D black-blood sequences, particularly 3D turbo spin-echo (TSE) with variable refocusing flip angles, have been introduced to image either intracranial [3-5] or carotid [6, 7] arterial vessel walls and has proven to be reproducible for arterial vessel wall and plaque morphological measurements [8]. However, existing arterial vessel wall analysis is based on two-dimensional (2D) cross-sectional images reconstructed from the isotropic 3D images by neuroradiologist, which is time consuming and experience dependent. Therefore, it is expected that an automated analysis method based directly on 3D black-blood MR images will be easier to operate and more accurate. In this study, we aim to develop an automated method to analyze 3D intra- and extracranial arterial vessel wall images, including vessel centerline tracking, vessel straightened reformation, vessel wall segmentation, and morphological quantification.Methods
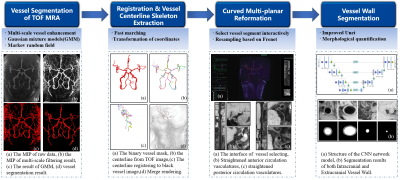
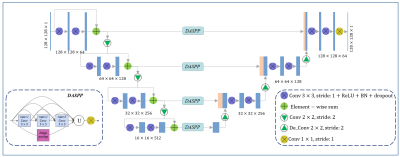
The automated analysis framework of arterial vessel wall morphology is built based on intra- and extracranial arterial vessel wall images acquired by recently developed T1-weighted DANTE-SPACE sequence [9] and TOF MRA images. TOF MRA is used to facilitate the tracking task at the location of small arterial segments, which is commonly challenging with only vessel wall images. The integrated workflow of image analysis is shown in Fig.1, along with example images at each stage. To begin with, comprehensive pattern recognition model is applied to vessel segmentation of TOF MRA. Next, the vessel centerline is extracted from the segmented TOF MRA using fast marching algorithm, and then co-registered to 3D vessel wall MR using coordinate transformation. After that, 3D curved multi-planar reformation (MPR) is employed to straighten the vessel of interest, and cross-sectional 2D slices of the vessel segment are obtained through resampling. Finally, the proposed U-net-like fully convolutional neural network (CNN) is applied to vessel wall segmentation (Fig.2), and then calculate the maximum wall thickness, wall and lumen areas, as well as normalized wall index (NWI). Dice coefficient was used to evaluate the overlapping ratio between the automated segmentation and manual ground truth. Also, two-tailed paired student’s t-tests were used for the comparison of quantification metrics derived from proposed method and manually reformatted.Results
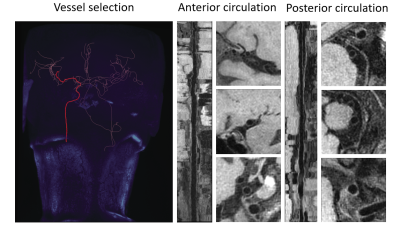
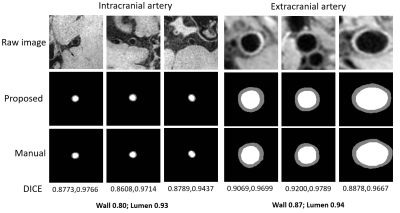
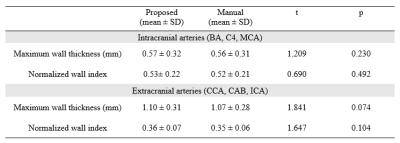
A total of 47 subjects completed the MR examinations and the proposed framework was used for image processing. Large-range anterior circulation (from internal carotid artery to M2 of the middle cerebral artery) and posterior circulation (from the vertebral artery to P1 of the posterior cerebral arteries) vasculatures were traced and straightened, 13680 2D slices of the interested vessel segments were generated. Representative processed images of a subject were presented in Fig. 3. Vessel wall can be clearly seen in both the longitudinal and transverse views, which largely facilitates the diagnosis of vessel wall diseases. The U-net-like network was trained and validated on 12960 slices and tested on 720 slices. The proposed segmentation method demonstrated satisfactory agreement with manual segmentation, as shown in Fig.4, the Dice coefficient of intracranial arteries was 0.93 for lumen and 0.80 for vessel wall while 0.94 for lumen and 0.87 for vessel wall of extracranial arteries. Moreover, our method was able to locate the vessel region and provide reasonable segmentation, even when the boundaries in neighboring tissues were essentially invisible to naked eyes. Tab.1 summarized the vessel wall morphological measurements of intra- and extracranial arteries from automated and manual methods, respectively on 20 random subjects. Although the maximum wall thickness and NWI of the proposed method were larger than the manual results for both intra- and extracranial arteries results, the differences are small and cannot be considered to be statistically significant (p > 0.05).Discussion and Conclusion
In this work, a framework to automatically segment the intra- and extracranial arterial vessel wall was developed, including functions of vessel path tracking, 3D MPR, vessel wall segmentation, and measurement calculation. The proposed method achieved good agreements with manual segmentation. The Dice coefficient of extracranial arteries, especially for the wall, was higher than that of intracranial arteries. This may be due to the possible leak at low-contrast outer wall boundary of smaller intracranial arteries. The Dice coefficient was slightly higher than previous studies [10], which benefited from the residual information added to preserve the original image features, and the spatial pyramid pooling used to extract multi-scale features of the proposed network. In conclusion, this automated analysis method makes large-scale quantitative vessel wall analysis easier and more accurate, which could potentially promote the adoption of Vessel Wall MRI in clinical application.Acknowledgements
This work was supported in part by National Natural Science Foundation of China (81830056, 81801691).References
1. G. Pasterkamp and E. Falk, “Atherosclerotic plaque rupture: An overview,” Journal of Clinical & Basic Cardiology, 2000. 3(2): p. 705–713.
2. C. A. Holmstedt, T. N. Turan, and M. I. Chimowitz, "Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment," Lancet Neurol, 2013. 12(11): pp. 1106-14.
3. Qiao, Y., D.A. Steinman, Q. Qin, M. Etesami, M. Schär, B.C. Astor, and B.A. Wasserman, Intracranial arterial wall imaging using three‐dimensional high isotropic resolution black blood MRI at 3.0 Tesla. Journal of Magnetic Resonance Imaging, 2011. 34(1): p. 22-30.
4. Fan, Z., Q. Yang, Z. Deng, Y. Li, X. Bi, S. Song, and D. Li, Whole‐brain intracranial vessel wall imaging at 3 T esla using cerebrospinal fluid–attenuated T1‐weighted 3 D turbo spin echo. Magnetic resonance in medicine, 2017. 77(3): p. 1142-1150.
5. Li, M.-L., Y.-Y. Xu, B. Hou, Z.-Y. Sun, H.-L. Zhou, Z.-Y. Jin, F. Feng, and W.-H. Xu, High-resolution intracranial vessel wall imaging using 3D CUBE T1 weighted sequence. European journal of radiology, 2016. 85(4): p. 803-807.
6. Wang, J., P. Börnert, H. Zhao, D.S. Hippe, X. Zhao, N. Balu, M.S. Ferguson, T.S. Hatsukami, J. Xu, and C. Yuan, Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magnetic resonance in medicine, 2013. 69(2): p. 337-345.
7. Fan, Z., Z. Zhang, Y.C. Chung, P. Weale, S. Zuehlsdorff, J. Carr, and D. Li, Carotid arterial wall MRI at 3T using 3D variable‐flip‐angle turbo spin‐echo (TSE) with flow‐sensitive dephasing (FSD). Journal of Magnetic Resonance Imaging, 2010. 31(3): p. 645-654.
8. Zhang, N., F. Zhang, Z. Deng, Q. Yang, M.A. Diniz, S.S. Song, K.H. Schlick, M.M. Maya, N. Gonzalez, D. Li, H. Zheng, X. Liu, Z. Fan, 3D whole-brain vessel wall cardiovascular magnetic resonance imaging: a study on the reliability in the quantification of intracranial vessel dimensions. Journal of Cardiovascular Magnetic Resonance, 2018. 20(1): p. 39.
9. Zhang, L., N. Zhang, J. Wu, X. Liu, and Y.-C. Chung, High resolution simultaneous imaging of intracranial and extracranial arterial wall with improved cerebrospinal fluid suppression. Magnetic resonance imaging, 2017. 44: p. 65-71.
10. Feng Shi , Qi Yang, Xiuhai Guo, Touseef Ahmad Qureshi, Zixiao Tian, Huijuan Miao , Damini Dey , Debiao Li , and Zhaoyang Fan , Intracranial Vessel Wall Segmentation Using Convolutional Neural Networks. IEEE Transactions on biomedical engineering, 2019. 66(10): p. 2840-2847.
Figures