1060
Network mapping of central post-stroke pain and analgesic neuromodulation1University Health Network, Toronto, ON, Canada, 2University Hospitals Leuven, Leuven, Belgium, 3GE Global Research, Bangalore, India
Synopsis
Despite the prevalence of central post-stroke pain (CPSP), pain-causing brain lesions remain incompletely understood. In 17 CPSP patients receiving invasive neuromodulation, we utilized voxelwise odds-ratio mapping and normative resting state fMRI to identify high-risk pain hotspots and describe functional networks associated with CPSP lesions and analgesic stimulation. Highest-risk CPSP hotspots were located in somatosensory thalamus/white matter and connected to a network comprising anterior cingulate cortex, insula, thalamus, and inferior parietal lobule. Posterior insula and thalamus were also coupled to therapeutic deep brain stimulation volumes. These findings elucidate CPSP’s topography and connectivity while informing the network-level mechanism of analgesic neuromodulation.
Introduction
Central post-stroke pain (CPSP) is a debilitating neuropathic pain syndrome that affects roughly 7.5% of stroke patients.1 Frequently refractory to pharmacotherapy, CPSP has been treated with mixed success using invasive neuromodulatory approaches such as deep brain stimulation (DBS) and motor cortex stimulation (MCS).2,3 Despite CPSP’s prevalence, the location of lesions most likely to cause pain and the identity of the wider functional networks that they impinge upon remains incompletely understood. We applied emerging neuroimaging techniques to a unique cohort of treatment-refractory CPSP patients undergoing DBS or MCS, aiming to (1) elucidate which brain areas most frequently incite pain when lesioned; (2) characterize functional networks associated with CPSP and analgesic neuromodulation.Methods
This multi-site study (Toronto Western Hospital, Canada & University Hospitals Leuven, Belgium) investigated 17 CPSP patients (10 females; age range: 37-81) with high-quality structural MR imaging who received either ventrocaudal thalamic nucleus/periventricular grey DBS (n=12) or MCS (n=5). Stereotactic targeting, surgical insertion, and programming and delivery of stimulation were performed as previously described for DBS and MCS.4,5T1-weighted imaging was obtained using a GE Signa Excite 1.5T scanner (Toronto: 3D-spoiled gradient echo; voxel size=1×1×1 mm, TR=12 ms, TE=5 ms, flip angle=20°) or Siemens Magnetom Expert 0.95T, Siemens Symphony/Sonata Vision 1.5T, Philips Ingenia 1.5T, or Philips Intera 3T scanner (Leuven: 3D-magnetization-prepared rapid acquisition gradient echo; voxel size=1×1×1 mm, TR=11 ms, TE=4 ms, flip angle=8°). All cerebrovascular lesions were manually segmented using pre-operative T1-weighted images and normalized to standard space (ICBM 2009b NLIN asymmetric) in a two-step process involving 12 DOF linear FLIRT and nonlinear FNIRT registration and employing the lesion masks as input-weighting volumes to minimize registration distortion.6 Electrode localization and volume of tissue activated (VTA) modelling were performed with Lead-DBS (https://www.lead-dbs.org/) for DBS patients who received pain relief from stimulation at last follow-up (n=5).
To identify high-risk pain hotspots in the brain, the locations of CPSP lesions and 220 control lesions from a publicly available stroke database (http://fcon_1000.projects.nitrc.org/indi/retro/atlas.html) were compared using voxelwise odds-ratio (VOR) mapping.7,8 Whole-brain voxelwise logistic regression (corrected for multiple comparisons using false discovery rate; FDR) comparing CPSP and control lesions was also performed in order to statistically validate and reinforce the VOR results. The functional connectivity of these hotspots and individual CPSP lesions was then investigated using a large (n=1000) normative resting state functional MRI connectome constructed from the Genomics Superstruct Project dataset (https://dataverse.harvard.edu/dataverse/GSP).9 This generated whole-brain connectivity r-maps (subsequently converted to t-maps) that reflected time course-dependent correlations between the seed and every voxel in the brain (in-house MATLAB script, The MathWorks, Inc., Version R2017b. Natick, MA, USA). Similar connectivity maps were obtained for the control lesions and therapeutic DBS VTAs.
In order to discern brain regions with meaningful connectivity to the seed and correct for multiple comparisons, connectivity t-maps were thresholded by t=5.1 (PBonferroni<0.05). In the case of t-maps obtained for individual lesions and DBS VTAs, the maps in each group were combined into an overall average map and masked by a sum map comprising only voxels that survived t-thresholding in ≥50% of individual connectomes.
To explore the specificity of CPSP lesion connectivity relative to that of non-pain-causing lesions, the unthresholded t-maps of the CPSP and control cohorts were compared using whole-brain voxelwise logistic regression. Finally, areas of connectivity common to both pain-causing lesions and therapeutic DBS were identified by creating a binarized overlap map that contained only voxels that appeared in both groups’ 50% frequency-masked average t-maps. All statistical analyses were performed using R (R 3.4.4, https://www.r-project.org) and RMINC (https://github.com/Mouse-Imaging-Centre/RMINC) software.
Results
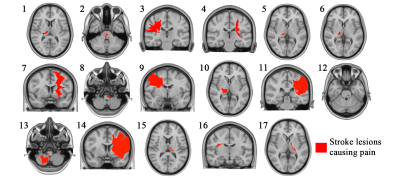
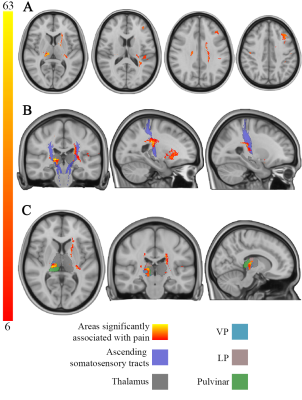
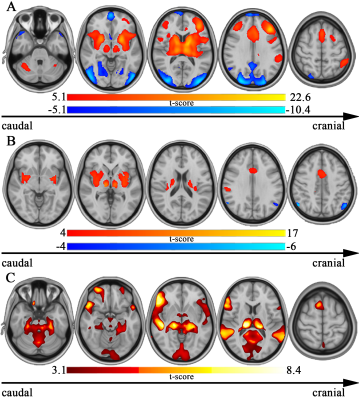
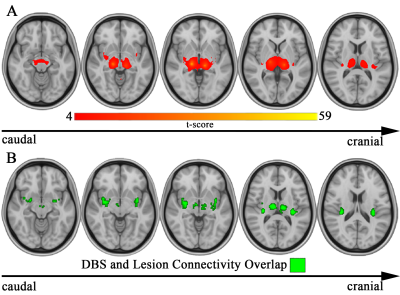
Pain-causing lesions were broadly distributed across the brain, encompassing both cortical and subcortical regions (Figure 1). Brain regions associated with greatest risk of CPSP (highest value=63 times greater risk) were located along the ascending somatosensory apparatus within thalamus (specifically the ventral posterior nucleus-pulvinar border), posterior limb of internal capsule, and corona radiata (Figure 2). These same areas were also significantly associated (PFDR<0.05) with CPSP based on logistic regression analysis. High-risk pain hotspots and the majority of individual CPSP lesions were functionally connected to anterior cingulate cortex, insula, thalamus, and inferior parietal lobule (PBonferroni<0.05) (Figure 3A,B). Connectivity to areas such as thalamus, inferior parietal lobule, and precuneus also differed between CPSP and control lesions (PFDR<0.05) (Figure 3C). Posterior insula and thalamus shared significant connectivity (PBonferroni<0.05) with both CPSP lesions and pain-alleviating DBS VTAs (Figure 4).Discussion
Expanding on earlier thalamus-specific VOR work,7 we identified high-risk brain regions for CPSP within somatosensory thalamus and along the length of the ascending somatosensory tracts. Our normative connectomics results indicate that CPSP hotspots and lesions engage a network of sensory, associative, and limbic regions previously implicated in both normal pain processing and chronic pain states10–12 and further suggest that thalamus and posterior insula might constitute hubs at which stimulation acts to normalize aberrant pain networks.Conclusion
These findings, derived from lesion mapping and state-of-the-art large-scale connectomics, inform the topography and functional connectivity of CPSP and provide insight into the network-level mechanism of analgesic neuromodulation.Acknowledgements
This study was supported by the RR Tasker Chair in Functional Neurosurgery at University Health Network and a Tier 1 Canada Research Chair in Neuroscience.
AML is a consultant for Medtronic, St Jude-Abbott, and Boston Scientific. SJ is an employee of GE Global Research.
References
1. Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain. 1995 May;61(2):187–93.
2. Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009 Sep;8(9):857–68.
3. Hosomi K, Seymour B, Saitoh Y. Modulating the pain network--neurostimulation for central poststroke pain. Nat Rev Neurol. 2015 May;11(5):290–9.
4. Hamani C, Schwalb JM, Rezai AR, Dostrovsky CO, Davis KD, Lozano AM. Deep brain stimulation for chronic neuropathic pain: long‐term outcome and the incidence of insertional effect. Pain. 2006;125:188–96.
5. Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993 Mar;78(3).
6. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002 Oct;17(2):825–41.
7. Sprenger T, Seifert CL, Valet M, Andreou AP, Foerschler A, Zimmer C, et al. Assessing the risk of central post-stroke pain of thalamic origin by lesion mapping. Brain. 2012 Aug;135(8):2536–45.
8. Boutet A, Ranjan M, Zhong J, Germann J, Xu D, Schwartz ML, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain. 2018 Dec 1;141(12):3405–14.
9. Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci. 2014 Oct 14;111(41):E4367–75.
10. Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013 Apr 11;368(15):1388–97.
11. Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010 Aug;62(8):2545–55.
12. Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS, et al. Network localization of neurological symptoms from focal brain lesions. Brain J Neurol. 2015 Oct;138(Pt 10):3061–75.
Figures