1059
Treatment efficacy of asymptomatic carotid artery stenosis patients evaluated by clinically applicable hemodynamic MRI and cognitive testing1School of Medicine, Department of Neuroradiology, Technical University of Munich, Munich, Germany, 2MRRC, Yale University, New Haven, CT, United States, 3School of Medicine, Department of Radiology, Technical University of Munich, Munich, Germany, 4Institute of Radiopharmaceutical Cancer Research, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 5Philips Healthcare, Hamburg, Germany, 6Institute of Clinical Medicine, Aarhus University, Aarhus, Denmark, 7School of Medicine, Clinic of Neurology, Technical University of Munich, Munich, Germany
Synopsis
Hemodynamic MRI is highly promising to improve treatment decisions in asymptomatic internal carotid artery stenosis (ICAS). However, treatment efficacy evaluations require clinically applicable techniques, such as dynamic susceptibility contrast (DSC) and resting-state BOLD-based evaluations of amplitude of low-frequency fluctuations (ALFF). We present data from 16 asymptomatic ICAS patients before and after treatment and 17 age-matched healthy controls measuring cerebral blood volume (CBV) and capillary transit-time heterogeneity (CTH) by DSC and ALFF with additional cognitive testing. We hypothesized recovery of hemodynamic impairments after revascularization. Our results confirmed this hypothesis for all parameters. Interestingly, at the same time cognitive function remained impaired.
Purpose
Internal carotid-artery stenosis (ICAS) is a major public health issue and accounts for approximately 10% of all strokes.1 While effective interventions by carotid artery stenting (CAS) or carotid endarterectomy (CEA) can reduce stroke risks,2 they come with substantial risks.3 Those competing risks complicate decisions, especially in asymptomatic ICAS and create the need for improved postoperative outcome evaluations.3 Even though hemodynamic-MRI4-6 is highly promising for this purpose,7,8 clinically applicable methods are missing.Individual stroke risk assessment was proposed by cerebrovascular reactivity (CVR) measurements.9-11 CVR was demonstrated to correlate with readily applicable amplitudes of low-frequency fluctuations (ALFF) of resting-state BOLD, based on spontaneous arterial CO2 fluctuations during normal breathing.8,12-14 CVR decreases in ICAS are caused by chronic vasodilation, inducing concomitant cerebral blood volume (CBV) increases.15-17 CBV imaging is clinically established by dynamic susceptibility contrast (DSC)-MRI.18 Recent DSC-modeling additionally derives capillary transit-time heterogeneity (CTH)19 and previous studies already indicated widespread capillary dysfunction in ICAS.20,21 However, further validations of ALFF and DSC-imaging are required to test their sensitivity to hemodynamic recovery after ICAS-treatment. Moreover, revascularization effects on known cognitive impairments5,22 are widely unknown.23,24
The aim of our study was therefore to evaluate treatment efficacy by ALFF and DSC-MRI in asymptomatic ICAS-patients and age-matched healthy controls (HC). We hypothesized recovery of hemodynamic impairments after treatment. Moreover, we investigated revascularization effects on cognitive performance.
Methods
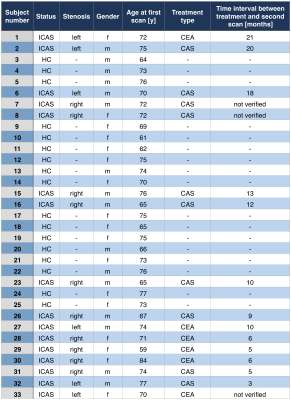
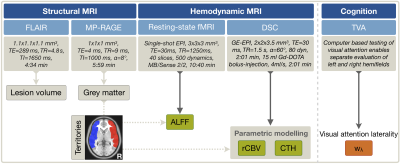
Thirty-three participants (16 asymptomatic, unilateral ICAS-patients, age=71.4±5.8y, mean NASCET 81% and 17 HCs, age=70.8±5.3y) underwent MRI on a 3T Philips Ingenia (Philips Healthcare, Best, Netherlands) using a 16-channel head-neck-coil. A second MRI-scan was performed in patients (follow-up time after treatment: minimum 3 months, mean 10.5 months, Tab.1) and in HC for comparisons. Measurements and derived parameters are summarized in Figure 1. On FLAIR, white matter lesion (WML) masks were segmented automatically25 and Fazekas-scores26 evaluated (JG,LS). ALFF was calculated with temporal filtering (0.01-0.08 Hz),27 after motion correction, careful artefact reduction by principal component analysis and smoothing (Gaussian FWHM=8x8x8mm3). Parametric modeling of DSC yielded relative CBV (rCBV) and CTH-maps.19 All parameter-maps were MNI-normalized. Processing was performed with SPM1217 and custom Matlab-programs. By visual ratings, artefact-affected maps were excluded (SK,CP). Median parameter-values between hemispheres were extracted in GM of MCA perfusion-territories28 (Fig.1),5,29 in ICAS ipsilateral and contralateral to the stenosis. Visual attention lateralization was tested based on the theory of visual attention (TVA).30 Two-sample t-tests were considered statistically significant at p<0.05.Results
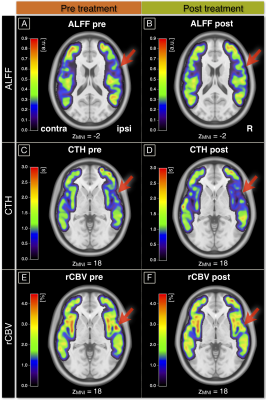
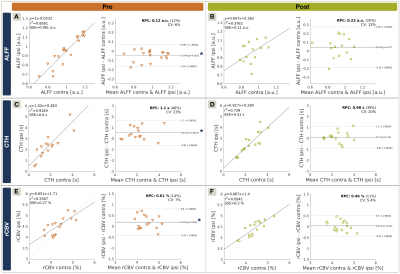
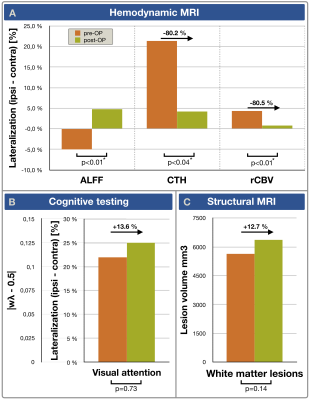
Exemplary data is shown in Figure 2. On group level, all hemodynamic parameters were impaired in ICAS before treatment (Fig.3A,C,E). ALFF was ipsilaterally decreased by -5.0% (p<0.01), CTH increased by +21.4% and rCBV increased by +4.3% (p<0.01 & p<0.03, respectively). Parameter lateralization ∆CTH and ∆rCBV was significantly stronger in ICAS compared to HC (p<0.001 & p<0.03), but not for ∆ALFF (p=0.07). After treatment, hemodynamic impairments recovered (Fig.3B,D,F). Lateralization ∆CTH and ∆rCBV improved by approximately 80%, each (Fig.4A). Symmetry was found for ALFF, rCBV and CTH (p>0.17, each). However, ALFF standard deviation increased (Fig.3B). At the same time, all parameters were symmetrical in both HC scans (data not shown). WML evaluation revealed no group differences before treatment (ICAS: 5648 mm3 vs. HC: 5033 mm3, p=0.66). After treatment, WML-load slightly increased non-significantly (ICAS: +718 mm3 with p=0.14, Fig.4C; HC: +297 mm3 with p=0.21). Fazekas-scores were unaffected in follow-up scans (ICAS: 1.56±0.63; HC: 1.12±0.93, in both scans, each).The cognitive performance of ICAS-patients before treatment was not globally affected,5 but visual attention lateralized by 22% (p<0.01). It was postoperatively unchanged (p=0.73) and remained lateralized (Fig.4B).
Discussion
As hypothesized, ALFF and DSC-based hemodynamic parameters were sensitive to impairments of ICAS in MCA-territories before treatment and their postoperative recovery, without compromising the specificity as affirmed by symmetrical HC results in both scans. In ICAS, ipsilateral ALFF decreases with concomitant rCBV increases indicate chronic vasodilation,4,17 which normalised after treatment, in agreement with the literature.31-33 Our measured long-term CTH recovery is in line with CTH recovery at 24h after treatment, as presented by Arsava et al.34In the presented patient cohort, we have previously demonstrated correlations of pre-OP lateralization between visual attention and perfusion.5 Despite of the postoperative hemodynamic improvements, we found unaffected cognitive performance, according with Schroeder et al.29 One possible explanation is that cognitive impairments are irreversible. Another possible explanation relates to associations of stenting with micro embolism risks, which can impair cognitive function.35 Cognitive decline by micro emboli35 and postoperative hyperfusion36 might thus compensate with improvements by normalized ∆CTH37. This would also fit with slightly increased postoperative WML.
ALFF variations were generally high and preoperative ∆ALFF=-5% was weak compared to previously presented ∆CVR=-20% by breath-hold fMRI in the same patient-cohort,38 pointing to limited sensitivity of ALFF in agreement with the literature.8,39
Conclusion
We successfully analyzed hemodynamic impairments and their recovery after treatment of ICAS-patients by ALFF and DSC-MRI in MCA-territories in line with previous studies. Both techniques are promising for clinical applications with reasonable scan times, availability at most MR-scanners and minimal required patient cooperation. However, diagnostics in individual patients may benefit from increased sensitivity of more sophisticated CVR-imaging techniques. Interestingly, we found unaffected cognition after revascularization. This implies irreversible effects even in asymptomatic ICAS and demands further evaluations in future studies to assess clinical ICAS-treatment outcomes and improve the treatment guidelines.Acknowledgements
We acknowledge support by the Friedrich-Ebert-Stiftung, Dr.-Ing. Leonhard-Lorenz-Stiftung (grant SK 971/19) and the German research Foundation (DFG, grant PR 1039/6-1).References
1. Petty GW, Brown RD, Jr., Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999;30:2513-6.
2. Demirel S, Böckler D, Storck M. Comparison of long-term results of carotid endarterectomy for asymptomatic carotid artery stenosis. Gefasschirurgie. 2018;23:1-7.
3. Alberts MJ. Carotid stenting—why treating an artery may not treat the patient. JAMA Neurology 2015;72:263-4.
4. Donahue MJ, Achten E, Cogswell PM, et al. Consensus statement on current and emerging methods for the diagnosis and evaluation of cerebrovascular disease. J Cereb Blood Flow Metab 2018;38:1391-417.
5. Gottler J, Kaczmarz S, Nuttall R, et al. The stronger one-sided relative hypoperfusion, the more pronounced ipsilateral spatial attentional bias in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2018:271678x18815790.
6. Gibbs JM, Leenders KL, Wise RJS, Jones T. EVALUATION OF CEREBRAL PERFUSION RESERVE IN PATIENTS WITH CAROTID-ARTERY OCCLUSION. The Lancet 1984;323:310-4.
7. Marshall RS, Lazar RM, Liebeskind DS, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis - Hemodynamics (CREST-H): Study design and rationale. Int J Stroke 2018;13:985-91.
8. Liu P, De Vis JB, Lu H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. Neuroimage 2019;187:104-15.
9. Donahue MJ, Dethrage LM, Faraco CC, et al. Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke 2014;45:2335-41.
10. Fisher JA, Venkatraghavan L, Mikulis DJ. Magnetic Resonance Imaging-Based Cerebrovascular Reactivity and Hemodynamic Reserve. Stroke 2018;49:2011-8.
11. Gupta A, Chazen JL, Hartman M, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 2012;43:2884-91.
12. Kazan SM, Huber L, Flandin G, Ivanov D, Bandettini P, Weiskopf N. Physiological basis of vascular autocalibration (VasA): Comparison to hypercapnia calibration methods. Magn Reson Med 2016.
13. Kannurpatti SS, Biswal BB. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. Neuroimage 2008;40:1567-74.
14. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537-41.
15. Derdeyn CP, Videen TO, Yundt KD, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain 2002;125:595-607.
16. Donahue MJ, Sideso E, MacIntosh BJ, Kennedy J, Handa A, Jezzard P. Absolute arterial cerebral blood volume quantification using inflow vascular-space-occupancy with dynamic subtraction magnetic resonance imaging. J Cereb Blood Flow Metab 2010;30:1329-42.
17. Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol 2009;30:876-84.
18. Welker K, Boxerman J, Kalnin A, Kaufmann T, Shiroishi M, Wintermark M. ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. AJNR Am J Neuroradiol 2015;36:E41-51.
19. Jespersen SN, Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab 2012;32:264-77.
20. Kaczmarz S, Gottler J, Petr J, et al. Hemodynamic impairments in asymptomatic unilateral carotid artery stenosis are increased within individual watershed areas. Proc Intl Soc Mag Reson Med 27; 2019; Montréal, Canada.
21. Mundiyanapurath S, Ringleb PA, Diatschuk S, et al. Capillary Transit Time Heterogeneity Is Associated with Modified Rankin Scale Score at Discharge in Patients with Bilateral High Grade Internal Carotid Artery Stenosis. PLoS One 2016;11:e0158148.
22. Lal BK, Dux MC, Sikdar S, et al. Asymptomatic carotid stenosis is associated with cognitive impairment. J Vasc Surg 2017;66:1083-92.
23. De Rango P, Caso V, Leys D, Paciaroni M, Lenti M, Cao P. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke 2008;39:3116-27.
24. Norling AM, Marshall RS, Pavol MA, et al. Is Hemispheric Hypoperfusion a Treatable Cause of Cognitive Impairment? Curr Cardiol Rep 2019;21:4.
25. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage 2012;59:3774-83.
26. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351-6.
27. Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 2008;172:137-41.
28. Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology 1998;50:1699-708.
29. Schroder J, Heinze M, Gunther M, et al. Dynamics of brain perfusion and cognitive performance in revascularization of carotid artery stenosis. Neuroimage Clin 2019;22:101779.
30. Bundesen C, Habekost T, Kyllingsbaek S. A neural theory of visual attention: bridging cognition and neurophysiology. Psychol Rev 2005;112:291-328.
31. Mandell DM, Han JS, Poublanc J, et al. Quantitative measurement of cerebrovascular reactivity by blood oxygen level-dependent MR imaging in patients with intracranial stenosis: preoperative cerebrovascular reactivity predicts the effect of extracranial-intracranial bypass surgery. AJNR Am J Neuroradiol 2011;32:721-7.
32. Sam K, Poublanc J, Sobczyk O, et al. Assessing the effect of unilateral cerebral revascularisation on the vascular reactivity of the non-intervened hemisphere: a retrospective observational study. BMJ Open 2015;5:e006014.
33. Fierstra J, Maclean DB, Fisher JA, et al. Surgical revascularization reverses cerebral cortical thinning in patients with severe cerebrovascular steno-occlusive disease. Stroke 2011;42:1631-7.
34. Arsava EM, Hansen MB, Kaplan B, et al. The effect of carotid artery stenting on capillary transit time heterogeneity in patients with carotid artery stenosis. Eur Stroke J 2018;3:263-71.
35. Maggio P, Altamura C, Lupoi D, et al. The Role of White Matter Damage in the Risk of Periprocedural Diffusion-Weighted Lesions after Carotid Artery Stenting. Cerebrovasc Dis Extra 2017;7:1-8.
36. Nanba T, Ogasawara K, Nishimoto H, et al. Postoperative cerebral white matter damage associated with cerebral hyperperfusion and cognitive impairment after carotid endarterectomy: a diffusion tensor magnetic resonance imaging study. Cerebrovasc Dis 2012;34:358-67.
37. Ostergaard L, Jespersen SN, Engedahl T, et al. Capillary dysfunction: its detection and causative role in dementias and stroke. Curr Neurol Neurosci Rep 2015;15:37.
38. Kaczmarz S, Gottler J, Sollmann N, et al. Recovery of cerebrovascular reactivity after asymptomatic carotid artery stenosis treatment is assessable by Breathhold-fMRI within global watershed areas. Proc Intl Soc Mag Reson Med 27; 2019; Montréal, Canada.
39. De Vis JB, Bhogal AA, Hendrikse J, Petersen ET, Siero JCW. Effect sizes of BOLD CVR, resting-state signal fluctuations and time delay measures for the assessment of hemodynamic impairment in carotid occlusion patients. Neuroimage 2018;179:530-9.
Figures