1057
Amide Proton Transfer Magnetic Resonance Imaging detecting early Wallerian degeneration of the pyramidal tract after ischemic stroke1Department of Radiology, the First Affiliated Hospital of Dalian Medical University, Dalian, China, 2Phillips healthcare, China, China
Synopsis
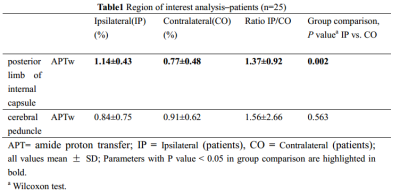
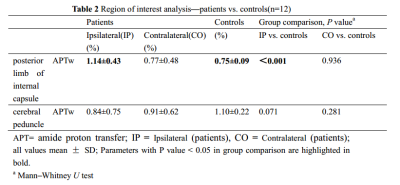
The study aimed to evaluate the significance of amide proton transfer weighted (APTw) imaging in the early diagnosis of pyramidal tract Wallerian degeneration (WD) following cerebral infarction. This study included 25 patients with acute cerebral infarction and 12 healthy adult controls. Our data suggested that the value of APTw was significantly increased on ipsilateral compared to contralateral and the control group in the posterior limb of internal capsule. It proves the feasibility of APTw in early detection of WD.
Introduction
Amide proton transfer (APT) imaging has recently emerged as an important contrast mechanism for magnetic resonance imaging (MRI) in the field of molecular and cellular imaging[1]. Wallerian degeneration (WD) is defined as progressive anterograde disintegration of axons and accompanying demyelination after an injury to the proximal axon or cell body[2,3]. The aim of this study was to evaluate the feasibility of APT imaging to detect the changes in early stage of Wallerian degeneration of the pyramidal tract after ischemic stroke.Methods
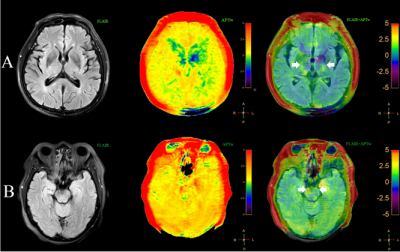
25 WD patients (15 men and 10 women; age range, 44-83 years) with middle cerebral artery stroke were examined 3-18 days after stroke and 12 age-matched normal controls (5 men and 7 women; age range, 48-74 years) underwent APT and traditional MRI examination on a Philips 3.0T MR scanner (Ingenia CX, Best, the Netherlands). Amide proton transfer weighted (APTw) images were collected with 3D-CEST-TSE sequence (7 slices, 2 x 2 x 7 mm3, B1=2 µT, tsat = 2s, , TA 6:50min). Regions of interest (ROI) in the posterior limb of internal capsule and cerebral peduncle were manually placed on FLAIR images based on anatomic knowledge about the location of the pyramidal tract (Figure 1). APTw measurements were performed in the posterior limb of internal capsule and cerebral peduncle. The Wilcoxon test for paired samples was used to evaluate the difference between the ipsilateral and contralateral within each patient. The Mann–Whitney U test for independent samples was also used to test the difference between two groups. This study has been approved by the local IRB.Results
There was no significant difference in age and sex between WD patients and normal control group. The value of APTw was significantly increased on the ipsilateral compared to the contralateral and the control group in the posterior limb of internal capsule (P=0.002, P<0.001, respectively). There was no significant difference of APTw in the cerebral peduncle between the ipsilateral and contralateral and between patients and controls (Table1 and 2).Discussion and Conclusion
Histopathological studies have shown that within the first days of WD, disintegration of axonal structures and fragmentation of the myelin sheaths can be observed[4]. In this study, our results have shown that the APTw signals intensity increased within a few days after the onset of ischemic stroke. The increase of free protein content in the process of demyelination may result in the increase of APTw signal intensity. Thus, the APTw are promising imaging biomarkers by which wallerian degenerantion at the molecular level can be assessed. However, there was no significant difference in the value of APTw in the cerebral peduncle in this study, which may be due to the fact that the anterograde disintegration of myelin sheath of WD failed to produce significant changes in the early distal pyramidal tract. However, more longitudinal studies are needed to confirm their causal relationship.Acknowledgements
No acknowledgement found.References
[1] Zhou J, Heo HY, Knutsson L, van Zijl P, Jiang S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J Magn Reson Imaging. 2019. 50(2): 347-364.
[2] Johnson, A.C., McNabb, A.R., Rossiter, R.J., 1950. Chemistry of Wallerian degeneration. A review of recent studies. Arch. Neurol. Psychiatry 64, 105–121.
[3] Lampert, P.W., Cressman, M.R., 1966. Fine-structural changes of myelin sheaths after axonal degeneration in the spinal cord of rats. Am. J. Pathol. 49, 1139–1155.
[4] Tricaud N, Park HT. Wallerian demyelination: chronicle of a cellular cataclysm. Cell Mol Life Sci. 2017. 74(22): 4049-4057.
Figures