1054
Stable fractional anisotropy and mean diffusivity after treatment in patients with asymptomatic high grade internal carotid artery stenosis1School of Medicine, Department of Neuroradiology, Technical University of Munich, Munich, Germany, 2MRRC, Yale University, New Haven, CT, United States, 3School of Medicine, Clinic of Neurology, Technical University of Munich, Munich, Germany, 4School of Medicine, Department of Radiology, Technical University of Munich, Munich, Germany
Synopsis
Internal carotid artery stenosis (ICAS) is a well-known risk factor for stroke. However, treatment efficacy evaluations are currently limited by widely unknown ICAS-effects on brain microstructure. Two promising parameters to study pathophysiological changes in white matter (WM) are fractional anisotropy (FA) and mean diffusivity (MD), derived from established diffusion-tensor imaging. We evaluated FA- and MD-maps in 15 ICAS-patients before and after revascularization. Our results demonstrated globally unimpaired FA and MD before treatment, even in watershed areas, which are especially vulnerable to hemodynamic impairments. Postinterventional structural results were stable. Thus, absence of ischemic events and successful treatment are indicated.
1. Purpose
Asymptomatic internal carotid artery stenosis (ICAS) is a common finding in the elderly population1 and also accounts for approximately 10% of all strokes.2,3 Yet effects of ICAS are diverse, ranging from hemodynamic impairments4 to reduced cognitive performances.5,6 In spite of its relevance, the pathophysiology of ICAS-related impairments is not entirely understood yet. Especially white matter integrity in asymptomatic ICAS patients after treatment is widely unknown.To measure white matter changes by magnetic resonance imaging (MRI) diffusion tensor imaging (DTI) is an established method7 and allows tracking of pathophysiological processes such as ischemic events.8 DTI derived parameters combined with information concerning perfusion are promising to identify asymptomatic patients, who are notably at risk, and also to monitor possible treatment effects. Enhanced information about structural effects in ICAS might prospectively improve treatment guidelines for asymptomatic patients to identify patients who benefit from invasive treatment.9,10
We addressed this issue in a follow-up study in patients with asymptomatic ICAS before and after revascularization treatment. We measured DTI-based fractional anisotropy (FA) and mean diffusivity (MD). Furthermore, we evaluated hemodynamic changes in terms of dynamic susceptibility contrast (DSC),11 deriving perfusion delays, i.e. time to peak (TTP) and individual watershed areas (iWSA),12 which are located at the junction of vascular territories and are known to be especially at risk at decreased perfusion pressure.
2. Methods
Fifteen patients (age at follow up 72.2±5.5 years, four females) with high grade unilateral asymptomatic ICAS (mean NASCET13 80%) were included in this follow-up study (mean time between baseline and follow-up scan: 12.2±5.5 months). Prior to the follow-up scan, patients underwent either carotid endarterectomy or carotid artery stenting (see Table 1 for patient details). MRI was performed on a clinical 3 Tesla Philips Ingenia (Philips, Best, The Netherlands), the scanning protocol contained MP-RAGE, DTI and DSC11 for both scans (see Fig. 1 for details of the imaging protocol). Whole brain examination of diffusion properties was performed using tract-based spatial statistics TBSS14 as implemented in FSL version 5.0.10 (FMRIB Software Library) with default parameters.15 To compare the ipsilateral and contralateral hemispheres, FA and MD maps of patients with left-sided stenosis were flipped in the mid sagittal axis. Subsequently, statistical analysis was performed using a paired t-test, applying 5000 random permutations and threshold-free cluster enhancement adjusted for age, gender, and stenosis degree. To enhance sensitivity for potentially minor structural changes, the diffusion parameters were also evaluated within the iWSAs for each patient before and after intervention. IWSA were semi-automatically defined based on DSC11 derived TTP maps.12 FA and MD values were extracted within these regions. In order to assess hemodynamics pre- and post-interventional, TTP values were normalized by the mean individual white matter TTP (relative TTP (rTTP)) and extracted within each iWSAs for additional between hemispheres. For this part of the analysis, SPM 1216 and custom MatLab17 programs were used.3. Results
Exemplary data and location of iWSA pre- and post-intervention are shown in Figure 2. Results of the whole brain TBSS analysis revealed no significant difference in a paired t-test between pre-interventional scan 1 (baseline) and post-interventional scan 2 (follow-up) for both, FA and MD (Fig. 3).Likewise, the more focused region of interest analysis of FA and MD values within iWSA showed no significant alterations between ipsilateral and contralateral hemispheres at both scans (Fig. 4b).
In the pre-interventional scan, rTTP values revealed significant difference between ipsilateral and contralateral hemispheres (Fig. 4a). In the post-interventional scan, no significant differences in the rTTP values between hemispheres were found, indicating normalized cerebral hemodynamics.
4. Discussion
We successfully defined iWSA in every patient and compared diffusion and perfusion parameters between hemispheres before and after revascularization treatment. In our patient cohort, hemodynamics differences between hemispheres were found within iWSA before intervention, but symmetry was restored after treatment (Fig. 4a).18 The examined diffusion parameters FA and MD were not affected at baseline or at follow-up (Fig. 4b). This was detected both, globally and within iWSA, indicating that revascularization procedures did not have relevant negative effects on brain integrity. While stroke prevention and risks connected to the procedure have to be regarded,19,20 the non-existence of ischemia after treatment suggests successful intervention. However, an improvement of diffusion such as an increase of FA has not been revealed either. This is controversial in the literature, as our findings are in line with the results of some previous studies,21 but opposing the findings of others that showed improvements of diffusivity measures following the procedure.22-24 This might be due to methodological differences or different patient cohorts in those studies, clearly indicating the demand for further investigation.5. Conclusion
In our longitudinal study, we considered information based on perfusion imaging and structural parameters in order to investigate the impact of ICAS in asymptomatic patients. Unchanged diffusion parameters and normalized perfusion underline success of revascularization treatment and absence of ischemia. To extend our knowledge on pathophysiological processes and refine treatment guidelines for asymptomatic patients, further studies in larger patient cohorts are required.Acknowledgements
The authors acknowledge support by the Dr.-Ing. Leonhard-Lorenz-Stiftung (grant SK 971/19) and the German research Foundation (DFG, grant PR 1039/6-1).References
1 de Weerd, M. et al. (2009). Prevalence of asymptomatic carotid artery stenosis according to age and sex: systematic review and metaregression analysis. Stroke, 40(4), 1105-1113.
2 D'Amore, C. et al. (2012). Border-zone and watershed infarctions. In Manifestations of Stroke (Vol. 30, pp. 181-184). Karger Publishers.
3 Jörgensen, L. et al. (1969). Ischaemic cerebrovascular diseases in an autopsy series part 2. Prevalence, location, pathogenesis, and clinical course of cerebral infarcts. Journal of the neurological sciences, 9(2), 285-320.
4 Kaczmarz S. et al. (2019). Hemodynamic impairments in asymptomatic unilateral carotid artery stenosis are increased within individual watershed areas. Proc. Intl. Soc. Mag. Reson. Med. 27 (2019): 3246.
5 Lal, B. K. et al. (2017). Asymptomatic carotid stenosis is associated with cognitive impairment. Journal of vascular surgery, 66(4), 1083-1092.
6 Göttler, J. et al. (2018). The stronger one-sided relative hypoperfusion, the more pronounced ipsilateral spatial attentional bias in patients with asymptomatic carotid stenosis. Journal of Cerebral Blood Flow & Metabolism, 0271678X18815790.
7 Le Bihan, D. et al. (2012). Diffusion MRI at 25: exploring brain tissue structure and function. Neuroimage, 61(2), 324-341.
8 Yang, Q. et al. (1999). Serial study of apparent diffusion coefficient and anisotropy in patients with acute stroke. Stroke, 30(11), 2382-2390.
9 Spence, J. D. et al. (2016). Appropriate management of asymptomatic carotid stenosis. Stroke and vascular neurology, 1(2), 64-71.
10 Marquardt, L. et al. (2010). Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke, 41(1), e11-e17.
11 Kluge, A. et al. (2016). Analysis of three leakage-correction methods for DSC-based measurement of relative cerebral blood volume with respect to heterogeneity in human gliomas. MRM, 34(4): p. 410-21.
12 Kaczmarz, S. et al. (2018). Increased variability of watershed areas in patients with high-grade carotid stenosis. Neuroradiology, 60(3): p. 311-323.
13 North American Symptomatic Carotid Endarterectomy Trial (1991) Methods, patient characteristics, and progress. Stroke 22:711–720.
14 Smith, S. M. et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 31(4), 1487-1505.
15 Woolrich, MW. et al. (2009) Bayesian analysis of neuroimaging data in FSL. Neuroimage; 45: S173–S186.
16 Statistical Parametric Mapping software (SPM12) Version 6225: www.fil.ion.ucl.ac.uk/spm.
17 MATLAB and Statistics Toolbox Release 2016b, The MathWorks, Inc., Natick, Massachusetts, United States.
18 Kaczmarz, S. et al. (2019). Recovery of cerebrovascular reactivity after asymptomatic carotid artery stenosis treatment is assessable by breathhold-fMRI within global watershed areas, Proc. Intl. Soc. Mag. Reson. Med. 27 (2019): 0739.
19 Young, K. C. et al. (2011). Does current practice in the United States of carotid artery stent placement benefit asymptomatic octogenarians? American Journal of Neuroradiology, 32(1), 170-173.
20 Jalbert, J. J. et al. (2015). Outcomes after carotid artery stenting in Medicare beneficiaries, 2005 to 2009. JAMA neurology, 72(3), 276-286.
21 Herweh, C. et al. (2012). Quantitative high-field diffusion tensor imaging of cerebral white matter in asymptomatic high-grade internal carotid artery stenosis. European neurology, 67(4), 246-251.
22 Sato, Y. et al. (2013). Postoperative increase in cerebral white matter fractional anisotropy on diffusion tensor magnetic resonance imaging is associated with cognitive improvement after uncomplicated carotid endarterectomy: tract-based spatial statistics analysis. Neurosurgery, 73(4), 592-599.
23 Soinne, L. et al. (2003). Brain diffusion changes in carotid occlusive disease treated with endarterectomy. Neurology, 61(8), 1061-1065.
24 Cheng, H. L. et al. (2012). Impairments in cognitive function and brain connectivity in severe asymptomatic carotid stenosis. Stroke, 43(10), 2567-2573.
Figures
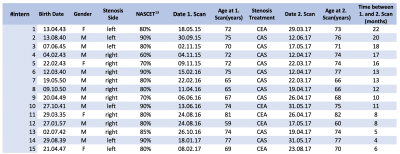
Table 1 – Overview clinical data
Summary of demographic patient data, including the side and severity of the stenosis, gender, age at the follow up in years, type of treatment (carotid endarterectomy (CEA) or carotid artery stenting (CAS)) and the interval between baseline and the follow up scan after treatment in months.
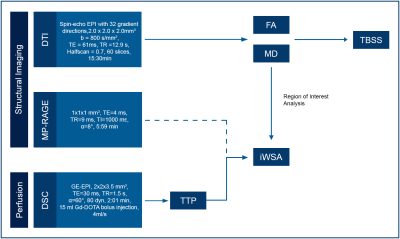
Figure 1 - MRI Protocol and Processing
The scanning protocol included for both scan times MP-RAGE and DSC, that were needed to define iWSAs. DTI delivered FA and MD maps. FA and MD were compared within iWSA for baseline and follow up scan. This part of the analysis was executed in the individual’s subject space while tract-based spatial statistics for global assessment of FA and MD was performed in MNI space.
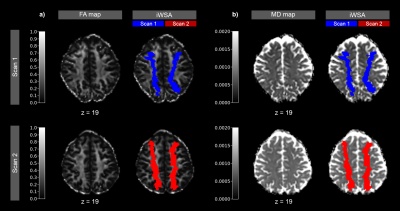
Figure 2 - Exemplary Data of a patient with right sided stenosis
FA (a) and MD maps (b) are shown for baseline and follow-up scan with color overlay of iWSAs at scan 1 (blue) and scan 2 (red). Note the shift of iWSAs after revascularization treatment in the second scan, indicating shift of vascular territories.
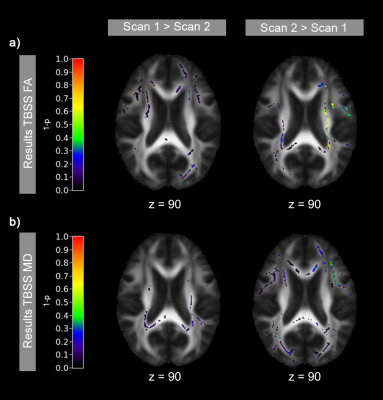
Figure 3 - Results of TBSS analysis
Scan 1 and 2 were compared using a paired t-test adjusted for age, gender and stenosis degree. Color overlays display results of two statistical comparisons: voxel values (FA (top) or MD (bottom)) in scan 1 > scan 2 (left column) and voxel values in scan 2 > 1 (right column). p-values are displayed as 1-p, so values above 0.95 are considered significant, which did not apply to any voxel.

Figure 4 –Paired Scatter Plots comparing single-subject rTTP and diffusion parameters within iWSA
Data points represent mean parameter-values of each subject and hemisphere within the iWSA. The line connects same subject’s corresponding hemisphere. The red dashed line display the group mean. a) TTP values were normalized by the mean individual white matter TTP to yield relative TTP (rTTP) and extracted within iWSA in white matter. b) and c) FA and MD values were extracted within iWSA and compared between ipsilateral and contralateral hemisphere.