1051
Developing a Novel and Robust Preprocessing Pipeline for Intensity-Based High-Resolution Magnetic Resonance Angiogram1Center for Magnetic Resonance Research, Department of Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
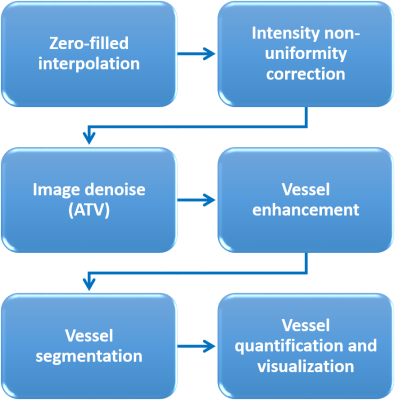
In-vivo high-resolution imaging of cerebral blood vessels is critical for brain functional research and clinical diagnosis. Despite well-developed magnetic resonance angiogram (MRA) techniques, a simple, robust preprocessing procedure has yet to be established. Thus, we propose a preprocessing pipeline that includes zero-fill interpolation, intensity non-uniformity correction, image denoising, vessel enhancement and segmentation. Specifically, we found that the most effective and robust denoising method is anisotropic total variation (ATV). By adopting and optimizing an improved 3D Hessian based tubular and spherical enhancement filter and a region-based level-set image segmentation method, we can automate the preprocessing of intensity-based MRAs with high fidelity.
Introduction
The ability to visualize vasculature is essential for clinical diagnosis and studying vascular anatomy and physiology. Magnetic resonance angiogram (MRA) based on the exogenous contrast agent and (or) the intrinsic blood property allows high-resolution in-vivo imaging of vessel structures, differentiating arteries and veins, and quantifying vessel density, size, and blood flow1-3. In spite of these advanced imaging techniques, there may be a lack of an efficient and automated vascular data-preprocessing procedure. To fill this gap, we propose a simple, fast, and robust pipeline to preprocess the intensity-based MRA to obtain denoised and segmented vessel images for further quantification. This pipeline includes following steps: i) image reconstruction of zero-filled k-space data to reduce the partial volume averaging effect4; ii) intensity non-uniformity correction of the inhomogeneous radio frequency field if necessary2; iii) image denoising to enhance signal-to-noise ratio (SNR), and iv) vessel enhancement and segmentation. In addition, to boost SNR, image denoise should not introduce excessive blur or imaging artifacts that may impoverish subsequent vessel segmentation and quantification. In order to determine the best denoising algorithm, we tested and compared filters of Gaussian, Weiner, anisotropic total variation (ATV) minimization5,6, and non-local means7. For vessel enhancement, we adopted an improved 3D Hessian based tubular and spherical enhancement filter8 and for vessel segmentation, we utilized a robust and fast level set method9. The proposed pipeline was tested on 3D time-of-flight (TOF) MRA and T2*-weighted imaging with exogenous contrast agent on different animal species with stable and precise outputs.Materials and Methods
Animals and scan conditions. Rat and cat were scanned with the protocol approved by the University of Minnesota IACUC. Animals were anesthetized with isoflurane and mechanically ventilated during scans. An IV catheter was placed to allow the infusion of the Feraheme as the contrast agent. A 1.5 cm diameter surface coil was placed on the top of the animal brains to maximize SNR. The physiology of the animals was monitored and well-controlled throughout the study.MRI experiments and data analysis. MRI was performed on a 9.4T/31cm animal scanner (Varian/VNMRJ). TOF MRA was acquired using 3D gradient-echo images with TR/TE = 32/5.5ms, FOV = 20×20x5 mm, matrix = 256x256x32, readout bandwidth = 37kHz, flip angle = 30°, 8 averages, total scan time ~35 mins. T2* images after Feraheme injection were acquired using the same parameters but with a reduced flip angle of 10° to maximize cortical signal. Images were preprocessed following the pipeline shown in Fig. 1 using MATLAB. Gaussian, Weiner, anisotropic total variation minimization, and non-local means algorithms were compared for their denoising effect. The segmented vessel images were used to differentiate arteries and veins. Quantification of vessel densities and sizes was performed.
Results
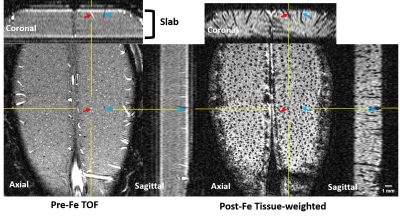
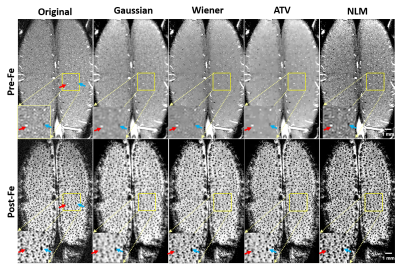
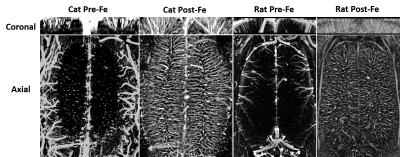
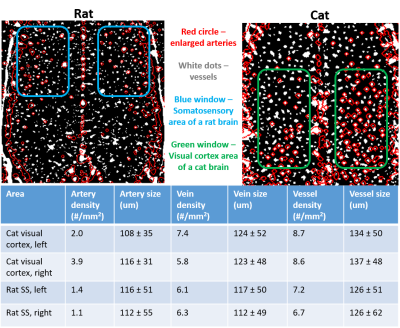
Fig. 2 shows the zero-filled high-resolution (39 x 39 x 78 μm) TOF MRA (Left) and T2* images after Feraheme injection (right), respectively. Tissue and blood inside the excited slab were dark due to the signal saturation while unsaturated blood coming from outside of the slab appears bright (see the red arrows). Note some of large veins appear dark because of the BOLD effect associated with a high concentration of deoxy-hemoglobin1. After injecting super-paramagnetic Feraheme, the intravascular signals dropped dramatically due to the reduced T2*, thus, all vessels appeared dark. The strong susceptibility effect also made vessel size appear larger than that of pre-Feraheme TOF. By comparing different denoising filters, we found ATV performed the best in terms of image restoration quality and visualization, especially for the pre-Feraheme TOF images as shown in Fig. 3. ATV-denoised vessel images were further enhanced and visualized through maximum intensity projection (MIP) to axial and coronal planes as shown in Fig. 4. The pre-Fe MIP images generally show the artery distribution while the post-Fe MIP images show a distribution of all vessels. Finally, image segmentation was performed to distinguish the arteries from veins by subtracting segmented Pre-Fe TOF from segmented post-Fe T2* images (see Fig. 5). Focusing on visual cortex of a cat and somatosensory (SS) area of a rat, we calculated the density and size of arteries and veins in left and right hemispheres. On average, sizes of artery and vein were 112 μm and 123 μm in cat visual cortex, and 114 μm and 115 μm in rat somatosensory areaDiscussion and conclusion
We proposed and tested a simple and robust preprocessing pipeline for intensity-based MRA analysis on high-resolution neurovascular images of animal brains. ATV-based image denoising leads to stable and precise results because it could remove the background interferences that have high variations while preserving the vessel information of interest, especially for pre-Fe TOF images. Other denoising methods will either introduce blur or enhance unwanted tissue structures. The region-based level set method accounts for the intensity inhomogeneity that is usually the case for TOF MRA where the vessel signal varies with vessel sizes and flow depth9. Finally, the measured artery size is close to real vascular size, though the size of veins is approaching the upper boundary. In summary, the proposed preprocessing pipeline for intensity-based MRA analysis is simple and robust and it produces high-fidelity and very high-resolution vessel images which should be highly valuable for studying angiogram distribution in the brain under healthy and diseased conditions.Acknowledgements
This work was supported in part by NIH grants of R01 MH111413, R01 CA240953, U01 EB026978, S10 RR025031, P41 EB027061 and P30 NS076408.References
1. Bolan, P. J., Yacoub, E., Garwood, M., Ugurbil, K. & Harel, N. In vivo micro-MRI of intracortical neurovasculature. Neuroimage 32, 62-69, doi:10.1016/j.neuroimage.2006.03.027 (2006).
2. Park, S. H., Masamoto, K., Hendrich, K., Kanno, T. & Kim, S. G. Imaging brain vasculature with BOLD microscopy: MR detection limits determined by in vivo two-photon microscopy. Magnet Reson Med 59, 855-865, doi:10.1002/mrm.21573 (2008).
3. Huang, C. H. et al. High-resolution structural and functional assessments of cerebral microvasculature using 3D Gas DeltaR2*-mMRA. PLoS One 8, e78186, doi:10.1371/journal.pone.0078186 (2013).
4. Du, Y. P., Parker, D. L., Davis, W. L. & Cao, G. Reduction of partial‐volume artifacts with zero‐filled interpolation in three‐dimensional MR angiography. Journal of Magnetic Resonance Imaging 4, 733-741 (1994).
5. Micchelli, C. A., Shen, L. X. & Xu, Y. S. Proximity algorithms for image models: denoising. Inverse Probl 27
6. Micchelli, C. A., Shen, L. X., Xu, Y. S. & Zeng, X. Y. Proximity algorithms for the L1/TV image denoising model. Adv Comput Math 38, 401-426, doi:10.1007/s10444-011-9243-y (2013).
7. Buades, A., Coll, B. & Morel, J. M. A non-local algorithm for image denoising. Proc Cvpr Ieee, 60-65 (2005).
8. Jerman, T., Pernus, F., Likar, B. & Spiclin, Z. Enhancement of Vascular Structures in 3D and 2D Angiographic Images. Ieee T Med Imaging 35, 2107-2118, doi:10.1109/Tmi.2016.2550102 (2016).
9. Li, C. et al. A level set method for image segmentation in the presence of intensity inhomogeneities with application to MRI. IEEE Trans Image Process 20, 2007-2016, doi:10.1109/TIP.2011.2146190 (2011).
Figures