1043
Event-Based Traversing of Hierarchical Sequences Allows Real-Time Execution and Arbitrary Looping in a Scanner-Independent MRI Framework1MR Physics, Fraunhofer MEVIS, Bremen, Germany, 2MR-Imaging & Spectroscopy, Faculty 01 (Physics/Electrical Engineering), University of Bremen, Bremen, Germany
Synopsis
MR sequence development is either based on complex, platform-specific solutions or restricted by fixed sequence structures together with a strict hierarchical implementation of loops. This abstract introduces event-based traversing of gammaSTAR sequences together with a buffered real-time execution at the scanner. The concept provides easy implementation of arbitrary, interleaved loop structures as well as memory efficient, real-time capable sequence execution in a vendor-agnostic environment. It is demonstrated for interleaved loop structures in a pCASL 3D GRASE sequence for brain perfusion imaging.
Introduction
The implementation of MR sequences is typically based on complex, platform-specific solutions and requires the definition of pre-defined execution orders and hierarchical loop structures that do not allow for easy implementation of arbitrary, interleaved sequence loops. In the recent years, the dynamic sequence development framework gammaSTAR (γ*) was developed [1-2]. In contrast to other platform-independent solutions [3-7] as well as the scanner-specific frameworks, it offers a flexible sequence structure during sequence execution.Here, MR sequences are represented as dynamic, hierarchical sequence trees which are interpreted at runtime. The developed graph-based calculations allow sequence parameter definitions to be independent of calculation order. In the previous implementation, sequence execution required a pre-calculation of all start times prior to the scan, which comes with memory issues and missing flexibility, and limited the framework to only execute sequences with a low amount of pulse events. In this abstract, a way of traversing the sequence structure in real time during the scan is presented. The concept allows the interpretation of interleaved sequence loop structures as well as complex sequence changes during runtime. It is demonstrated for pseudo-continuous Arterial Spin Labeling (pCASL, [8]) in which the background suppression pulse loop and the pCASL labeling train are implemented using two dedicated, interleaved loop structures.
Methods
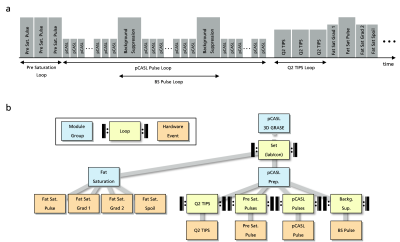
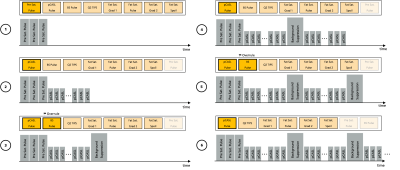
Figure 1a shows the chronological order of the hardware events of a pCASL preparation, highlighting the interleaved manner of the pCASL and background suppression (BS) pulse loops. User-friendly implementation would require both sequence elements to be executed by independent, interleaved sequence loops with automatic interpretation of overlapping hardware events. Figure 1b shows a hierarchical gammaSTAR sequence structure in which these loops are implemented accordingly.Real-time sequence execution needs the sequence tree to be traversed in runtime to always collect the next hardware event to be executed. Traversing the tree in a strict hierarchical way with defined loop order, as done by conventional MR sequence development frameworks, would separate the execution of the pCASL and BS pulse loops and result in runtime errors due to negative start times when executing the later loop. To correctly traverse the sequence structure, the loops in gammaSTAR runtime execution are decoupled from the execution order of hardware events. Due to the graph-based calculations, the start times of all hardware events of the current loop states are known at each timepoint. The hardware events are sorted in order of execution and the next hardware event is chosen from this list. The start times are recalculated only when required such that a major part of the sequence can be cached for low computational cost. Loop definitions are only required to trigger recalculation of the start times and events when all related hardware events of a loop iteration are executed (see Figure 2). In the case of two overlapping hardware events, i.e. from two different loop structures, an event-specific flag is read to decide on-the-fly whether one of the events overrules the other or both events should be combined (3, 5).
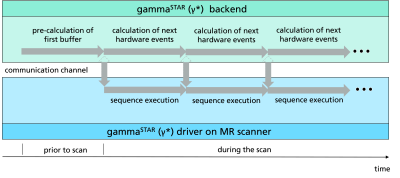
During the scan, the real-time sequence stream sends only a small number of pre-calculated hardware events to the MR scanner while traversing the sequence tree to find the subsequent events. To reduce computation time, these two processes can be run in separate threads on the scanner (see Figure 3). In the case of dynamic sequence changes throughout the scan, i.e. by feedback devices [9], a synchronization step can be executed for each buffer time (dashed arrows).
For evaluation of the buffered real-time sequence execution with on-the-fly interpretation of interleaved loops, brain perfusion of a healthy volunteer (male, 29) was acquired using a dedicated gammaSTAR pCASL 3D GRASE sequence at 3T (MAGNETOM Skyra, Siemens Healthineers AG). Sequence parameters were as follows: TR=4s, TE=42ms, FOV=256x256x64mm3, matrix size=64x64x16, EPI factor=64, turbo factor=8, partitions=16, repetitions=5. Because there is no feedback required, the synchronization step is disabled. The buffer time, i.e. the amount of hardware events to be pre-calculated, was chosen as low as possible without resulting in sequence abortion.
Results
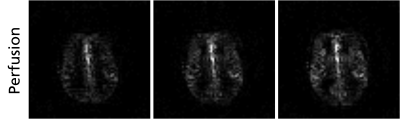
Buffer times as short as 100 ms were feasible when acquiring the described pCASL 3D GRASE sequence. In this time, up to 163 events of the total number of 46660 hardware events were pre-calculated before sending the tasks to the scanner hardware. The buffered real-time execution resulted in a reduction of allocated memory by a factor of 5.5. Figure 4 shows the perfusion-weighted images, acquired by the gammaSTAR framework.Discussion & Conclusion
This abstract demonstrated a way to execute gammaSTAR sequences in real time by chronologically traversing the sequence tree structure during sequence execution and pre-calculating only a small buffer of hardware events. This enables the acquisition of complex sequences with a high number of hardware events and interleaving loop structures at low memory and computation costs in a scanner-independent environment. The buffer concept also offers synchronization steps between interpretation of the sequence structure and executing the hardware events at the MR scanner that allows feedback devices to change sequence parameters and timings as it is required for interventional MRI or prospective motion correction.Acknowledgements
This work was supported by the FhG Internal Programs (Grant No. Attract 142-600172). The authors are grateful to David Porter's contributions to the initial stage of the overall project and to Robin Wilke and Jörn Huber for valuable discussion and support.References
[1] Cordes C, Konstandin S, Porter D, Günther M. Portable and platform-independent MR pulse sequence programs. Magn Reson Med (in print). doi: 10.1002/mrm.28020.
[2] https://gamma-star.mevis.fraunhofer.de
[3] Jochimsen TH & von Mengershausen M. ODIN-object-oriented development interface for NMR. J Magn Reson 2004;170(1):67-78.
[4] Stöcker T, Vahedipour K, Pfugfelder D, Shah NJ. High-performance computing MRI simulations. Magn Reson Med 2010;64(1):186-93.
[5] Magland JF, Li C, Langham MC, Wehrli FW. Pulse sequence programming in a dynamic visual environment: SequenceTree. Magn Reson Med 2016;75(1):257-65.
[6] Layton KJ, Kroboth S, Jia F, Littin S, Yu H, Leupold J, Nielsen JF, Stöcker T, Zaitsev M. Pulseq: A rapid and hardware‐independent pulse sequence prototyping framework. Magn Reson Med 2017;77(4):1544-52.
[7] Nielsen JF & Noll DC. TOPPE: A framework for rapid prototyping of MR pulse sequences. Magn Reson Med 2018;79(6):3128-34.
[8] Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008;60(6):1488-97.
[9] Hoinkiss DC, Cordes C, Konstandin S, Huber J, Wilke R, Günther M. Real-time Sequence control for prospective motion correction in a dynamic, platform-independent MRI framework. Proceedings of the ESMRMB 2019;S14.02.
Figures