1025
Organ-based estimation of the chronological age based on 3D MRI scans1University of Stuttgart, Stuttgart, Germany, 2University Hospital Tübingen, Tübingen, Germany
Synopsis
Age is an essential clinical parameter. It is often utilized as a risk factor for various disorders with the potential of influencing therapeutic decisions. However, a discrepancy exists between the chronological age (CA) and the biological age (BA) of an individual due to many factors such as medical history, genetics and lifestyle. In this preliminary work, we propose a novel deep-learning architecture for organ-specific CA estimation from 3D MR volumes for the brain and knee. We hypothesize that the introduced organ-specific approach would enable future analysis of the BA as different organs are expected to exhibit different aging characteristics.
Introduction
Age is one of the most important parameters describing individuals in a medical context. From an epidemiological point of view, age is a significant risk factor for frequent disorders including cardiovascular disease, cancer and neurodegenerative disorders1. Additionally, age is also an important parameter influencing therapeutic decisions. However, a large variation can be observed between the age-related effects described above and the average characteristics of the respective age group. Thus, it is important to distinguish between the chronological and the biological age. The chronological age (CA) is the amount of time passed since the birth of an individual. However, the biological age (BA) is not clearly defined since it reflects the actual ageing characteristics of an individual2. It follows that an individual's BA could deviate from his CA in an organ-dependant manner which depends on a manifold of factors such as lifestyle, genetics and medical history3-8.In this work, we propose a novel deep learning architecture for organ-specific CA estimation based on full 3D MR volumes. We apply the proposed network for the estimation of the brain and knee-joint age. Both are organs which exhibit relevant ageing characteristics. Quantitative comparisons were conduced against other state-of-the-art MR age-estimation frameworks.
Material and Methods
To achieve an accurate age-estimation performance, a new 3D regression architecture was designed based on a combination of the Inception v19 architecture and Fire modules from the SqueezeNet10 framework. This hybrid combination enables the design of a deeper and wider network while maintaining the same computation budget for 3D operations. The proposed architecture is composed of an initial stem network consisting of 3 convolutional layers followed by 4 consecutive modules, each composed of two inception blocks and 1 fire module. An overview of the proposed framework is depicted in Fig. 1.The proposed architecture was evaluated separately on the tasks of brain and knee-joint CA estimation. For the first task, axial T1-weighted brain MR scans were acquired for 550 anonymized patients from the open-source IXI brain dataset11. Scans had an original matrix size of 250x250x150. For pre-processing, the following procedure was followed12. First, the gray matter (GM) content was segmented using the statistical parameter mapping 12 (SPM12) software13. Then, the resultant GM tissues were normalized according to the ICBM 152 template and non-linearly registered and resampled using a 4 mm smoothing kernel14 resulting in a final matrix size of 121x145x121. For the knee-joint CA estimation, sagittal T2-weighted scans with fat suppression from 550 patients were acquired from the open-source NYU fastMRI dataset15. The data have an acquisition size of 320x320x28 and was normalized to have zero mean and unit variance. Each MR volume was split into individual 3D chucks of matrix size of 121x145x12 and 320x320x4 for the brain and knee, respectively, before being fed into the network. For both the brain and the knee age-estimation tasks, the patients were divided into groups of 415, 90 and 45 for training, cross-validation and testing, respectively. The data distribution for the utilized datasets is illustrated in Fig. 2 with additional input examples depicted in Fig. 3.
The performance of the proposed network was compared quantitatively against other state-of-the-art MR-based age regression networks with identical training hyperparameters. All networks were trained for 100 epochs using the ADAM optimizer. Namely, a 2D VGG-based architecture16 and a recent 3D-CNN framework17. The regression performance was evaluated using the mean absolute error (MAE), standard deviation (SD) and root mean square error (RSME) as metrics.
Results and Discussion
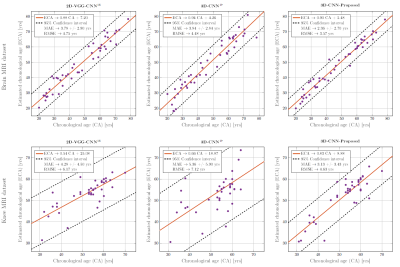
The quantitative results for the proposed model are presented in Table 1 and Fig. 4. The proposed hybrid 3D network is able to outperform the other existing frameworks by a significant margin. For instance, the resultant MAE have improved to 2.36±2.70 years compared to 3.83±2.84 years by the nearest competitor for brain age-estimation. The same quality of performance is also observed for the knee-joint age estimation. Also, we hypothesize that the brain exhibits better age-estimation performance compared to the knee-joint due to the availability of more recognizable age-related characteristics in the brain.To summarize, in this preliminary study we propose a new network architecture for organ-specific CA age estimation which outperforms concurrent state-of-the-art approaches. We hypothesize that conducting the age-estimation task in an organ-specific manner enables a precise tissue-specific CA estimation from which a potential BA (as the deviation from the mean age group) can be derived. In the future, this hypothesis will be investigated in-depth. In this case, different organs can be expected to yield different age scores depending on different factors such as medical history and lifestyle. Additionally, we would like to investigate whether brain BA age estimation could facilitate the early detection of neurogenerative disorders such as Alzheimer.
Conclusion
In this preliminary study, we proposed a novel 3D framework for organ-specific age estimation based on 3D MRI data. The framework was applied on two separate tasks, brain and knee-joint age estimation. To evaluate the proposed work, quantitative comparisons were conducted against state-of-the-art MR age estimations networks. A significantly improved performance was reported by the proposed framework. In the future, we would like to investigate the early detection of pathologies and disorders by analyzing cases were the estimated age deviates from a patient's known CA.Acknowledgements
No acknowledgement found.References
1: T. Niccoli, L. Partridge, "Ageing as a Risk Factor for Disease", Current Biology, vol. 22, no. 17, pp. R741-R752, 2012
2: L. Jia, W. Zhang, and X. Chen, "Common methods of biological age estimation.", Clinical Interventions in Aging, vol. 12, p. 759-772, 2017.
3: B.H. Chen, et al., "DNA methylation-based measures of biological age: meta-analysis predicting time to death.", Aging (Albany NY), vol. 8, no. 9, pp. 1844-1865, 2016.
4: V. Ignjatovic, et al., "Age-related differences in plasma proteins: how plasma proteins change from neonates to adults." PLoS One, vol. 6, no. 2, 2011.
5: J. Jylhävä, N.L. Pedersen, and S. Hägg, "Biological Age Predictors.", EBioMedicine, vol. 21, pp. 29-36, 2017.
6: E. Nakamura and K. Miyao, "A method for identifying biomarkers of aging and constructing an index of biological age in humans", The Journal of Gerontology: vol. 62, no. 10, pp- 1096-1105, 2017.
7: E. Albrecht, et al., "Telomere length in circulating leukocytes is associated with lung function and disease." European Respiratory Journal, vol. 43, no. 4, pp. 983-992, 2014.
8: J. Park, et al., "Developing a biological age assessment equation using principal component analysis and clinical biomarkers of aging in Korean men.", Archives of Gerontology and Geriatrics, vol. 49, no. 1, pp. 7-12, 2009.
9: J. Carreira and A. Zisserman. "Quo vadis, action recognition? new models and the kinetics dataset." In IEEE International Conference on Computer Vision and Pattern Recognition CVPR, pp.4724–4733, 2017.
10: F. N. Iandola, et al., “Squeezenet: Alexnet-level accuracy with 50x fewer parameters and <1mb model size,” arXiv pre-print, 2016.
11: "IXI Brain Dataset", avaliable: https://brain-development.org/ixi-dataset/.
12: C. D. Good, et al., “A voxel-based morphometric study of ageing in 465 normal adult human brains,” NeuroImage, vol. 14, no. 1, pp. 21 – 36, 2001.
13: "SPM12 software", available: https://www.fil.ion.ucl.ac.uk/spm/software/spm12/
14: J. Ashburner, “A fast diffeomorphic image registration algorithm,” NeuroImage, vol. 38, no. 1, pp. 95–113, 2007.
15: J. Zbontar, et al., “fastMRI: An open dataset and benchmarks for accelerated MRI,”arXiv preprint, 2018.
16: T. Huang, et al., “Age estimation from brain MRI images using deep learning,” in 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), pp. 849–852, 2017.
17: M. Ueda, et al., “An age estimation method using 3D-CNN from brain MRI images,” in 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), pp.380–383, 2019.
Figures