1018
OSSI Manifold Model for High-Resolution fMRI Joint Reconstruction and Quantification1Electrical Engineering and Computer Science, University of Michigan, Ann Arbor, MI, United States, 2Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States
Synopsis
Oscillating Steady-State Imaging (OSSI) is a new fMRI acquisition method that can provide high SNR signals, but does so at the expense of imaging time. We previously used a physics-based regularizer for high-quality, undersampled reconstruction by modeling the oscillating signal with physics parameters. However, the reconstructions were not quantitative, as the key parameter $$$T_2'$$$ for BOLD effects was not studied. In this work, to quantify MRI parameters of physiological importance, we jointly reconstruct the images and the parameters. The proposed manifold model reconstructs high-resolution images from 12-fold undersampled data, while also providing quantitative $$$T_2'$$$ estimates for fMRI.
Introduction
Oscillating Steady-State Imaging (OSSI) is a new fMRI acquisition method that has the potential to provide higher SNR than GRE imaging1. However, due to the oscillatory nature of the signal, multiple images with short TRs must be acquired and combined for fMRI analysis, potentially compromising the temporal resolution. Instead of imposing low-rankness or sparsity priors that may not suit fMRI data, we introduced a physics-based regularizer that models the generation of OSSI signals for reconstruction from undersampled data. However, the reconstructed images were unitless and were not quantitative in terms of important physiological related parameters2, especially for $$$T_2'$$$ that represents intravoxel dephasing due to BOLD effects. In this work, we construct a $$$T_2'$$$-weighted signal manifold and apply a near-manifold regularizer to jointly optimize OSSI images and quantitative maps. The manifold model enables a 12-fold acquisition acceleration and also provides accurate quantitative estimates for fMRI.Methods
In OSSI, the transverse magnetization of one isochromat lies on a physics-based manifold that is parameterized as$$m_0 \mathbf{\Phi}(T_1, T_2, T_2', f_0; t) \in \mathbb{C}^{n_c}\ : \ m_0 \in \mathbb{C}, \ T_1, T_2, T_2', f_0 \in \mathbb{R},$$where $$$m_0$$$ is the signal magnitude, $$$T_1$$$ and $$$T_2$$$ are the tissue parameters, $$$T_2'$$$ represents BOLD effects, $$$f_0$$$ is the off-resonance frequency, and $$$n_c$$$ is the number of TRs in an oscillation of OSSI.We simplify the manifold further because $$$T_1$$$ has primarily a scaling effect that we absorb into $$$m_0$$$, $$$t \approx$$$ TE for the short TR of OSSI, and we set $$$T_2$$$ to a textbook value. Therefore, our proposed approach for joint reconstruction and quantification using a manifold model regularizer becomes
$$\hat{\mathbf{X}},\hat{m_0},\hat{T_2'},\hat{f_0} =\underset{\mathbf{X},m_0,T_2',f_0}{\text{arg min}}\frac{1}{2}\lVert\mathcal{A}(\mathbf{X})-\mathbf{y} \rVert_2^2+ \beta \sum_{i,j} \lVert\mathbf{X}[i,j,:]-m_0 \mathbf{\Phi} (T_2',f_0)\rVert_2^2, \quad$$
where $$$\mathbf{X} \in \mathbb{C}^{N_x \times N_y \times n_c}$$$ denotes $$$n_c$$$ OSSI images to be reconstructed, $$$\mathbf{y}$$$ represents sparsely sampled k-space data, and $$$\mathcal{A}(\mathbf{\cdot})$$$ is a linear operator consisting of coil sensitivities, NUFFT, and an undersampling function. For each voxel $$$\mathbf{X}[i,j,:] \in \mathbb{C}^{n_c}$$$ is a vector of fast-time signal values, and $$$m_0 \mathbf{\Phi}(T_2',f_0) \in \mathbb{C}^{n_c}$$$ is the simplified manifold model. $$$\beta$$$ is the regularization parameter.
We solve the parameter estimation problem with VARPRO3 and a discrete realization of the manifold, which is a $$$T_2'$$$ signal dictionary formed with varying physics parameters. Specifically, we simulate the $$$T_2'$$$ signal for each central frequency $$$f_0$$$ by averaging complex signals from a set of isochromats with different off-resonance frequencies that are Cauchy distributed.
All the data were acquired on a 3T GE MR750 scanner with a 32-channel Nova Medical head coil. OSSI TR = 15 ms, $$$n_c$$$ = 10 TRs per signal oscillation, spiral-out TE = 2.7 ms, and flip angle = 10$$$^\circ$$$. The spatial resolution = 1.3$$$\times$$$1.3$$$\times$$$2.5 mm$$$^3$$$ for a 220 mm FOV, and the temporal resolution = 150 ms (after 2-norm combination of every $$$n_c$$$ OSSI images). We collected resolution phantom data with GRE imaging at varying TEs and estimated corresponding MRI parameters to validate the potential of using OSSI sequence and the proposed $$$T_2’$$$ manifold for quantification. For human data, the acceleration factor = 12 for both retrospective and prospective undersampling, and the sampling trajectory was a single-shot variable-density spiral with randomized rotations between frames. The functional task was a left/right reversing-checkerboard visual stimulus for 200 s (20 s L/20 s R $$$\times$$$ 5 cycles).
Results
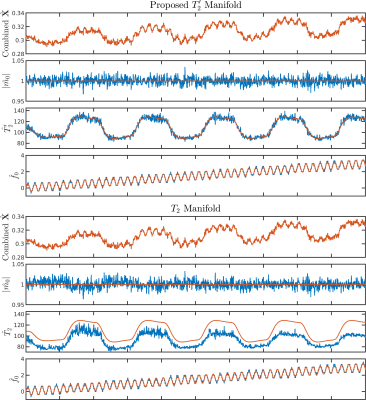
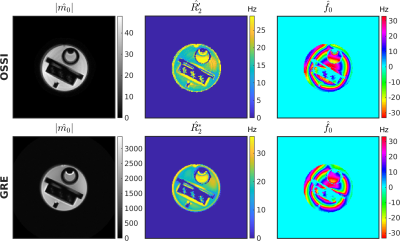
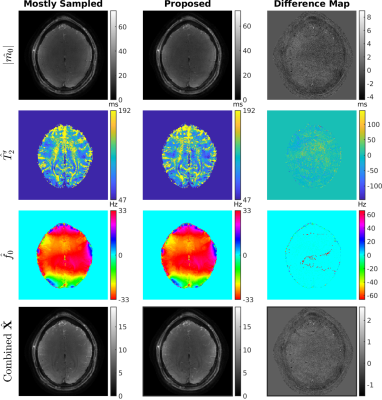
For one simulated voxel with functionals changes, $$$T_2’$$$ manifold results in more accurate parameter estimations compared to $$$T_2$$$ manifold (Figure 1). The resolution phantom qualifications demonstrate that OSSI manifold can be used for quantifying parameters and provides estimations comparable to multi-echo GRE imaging (Figure 2). The comparison of quantitative results from mostly sampled data and retrospectively undersampled data show that the proposed model almost fully recovers high-resolution structures and quantitative properties of the images from 8% of fully sampled k-space (Figure 3). The proposed manifold model jointly reconstructs high-resolution fMRI images and parameter maps with prospectively undersampled data (R = 12). The functional signals and the high SNR advantage of OSSI are well preserved (Figure 4).Conclusion
We present a $$$T_2’$$$ manifold to accurately model OSSI fMRI signals. Together with a near-manifold regularization for undersampled reconstruction, we are able to jointly optimize the high-resolution images and important fMRI parameters such as $$$T_2’$$$ with a factor of 12 acquisition acceleration.Acknowledgements
We wish to acknowledge the support of NIH Grants R01EB023618 and U01EB026977.References
1. Shouchang Guo and Douglas C. Noll, ''High SNR Functional MRI Using Oscillating Steady State Imaging". Joint Annual Meeting ISMRM-ESMRMB, Paris 2018, In Proc. Intl. Soc. Mag. Reson. Med.
2. Olafsson, Valur T., Douglas C. Noll, and Jeffrey A. Fessler. "Fast Joint Reconstruction of Dynamic $$$ R_2^* $$$ and Field Maps in Functional MRI." IEEE transactions on medical imaging 27.9 (2008): 1177-1188.
3. Golub, Gene, and Victor Pereyra. "Separable nonlinear least squares: the variable projection method and its applications." Inverse problems 19.2 (2003): R1.
Figures