1017
Model-based quantitative mapping for highly accelerated first-pass perfusion cardiac MRI1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Philips Healthcare, Guildford, United Kingdom
Synopsis
First-pass perfusion cardiac MR (FP-CMR) is becoming essential for evaluating myocardial ischemia. However, FP-CMR requires ECG-gating and breath-holding, leading to a trade-off between spatial resolution and coverage. Moreover, perfusion abnormalities are often identified visually by highly trained operators. Recently, quantitative FP-CMR and compressed sensing (CS) have been proposed to reduce operator-dependency and moderately accelerate acquisitions, respectively. Here, a model-based reconstruction is proposed to directly estimate quantitative myocardial perfusion maps from highly undersampled acquisitions. Thus, allowing for higher spatial resolution and coverage than indirect methods, where dynamic images are reconstructed using CS and quantitative maps are obtained subsequently using tracer-kinetic modeling.
Introduction
First-pass perfusion cardiac MR (FP-CMR) is one of the non-invasive tools of choice for evaluating coronary heart disease (CHD), the leading cause of death worldwide. However, FP-CMR requires ultra-fast acquisitions (to capture the first pass of a contrast bolus), ECG-gating and breath-holding to reduce cardiac and respiratory motion, leading to a trade-off between spatial resolution, heart coverage, false-positive defects due to dark-rim artifacts and motion induced artifacts.1,2 Moreover, perfusion abnormalities are often identified visually and thus, diagnostic accuracy is dependent on the level of training and experience of the operator.3 Recently, quantitative methods have been proposed to achieve an operator-independent assessment of myocardial perfusion.4,5 Typically, these methods indirectly generate quantitative myocardial perfusion maps by first reconstructing individual dynamic contrast-enhanced images, which are then converted to contrast agent concentration and finally, tracer-kinetic (TK) modeling is used to generate TK parameter maps. Moreover, compressed sensing (CS) and parallel imaging reconstruction methods have been proposed to generate dynamic images from moderately accelerated acquisitions as a means of improving spatial resolution.6-9 In this work, inspired by PET and dynamic contrast-enhanced MRI direct model-based parametric reconstruction methods,10-12 a DIRect QuanTitative (DIREQT) FP-CMR reconstruction method is proposed to directly estimate TK parameter maps from highly undersampled acquisitions, by exploiting the redundancy of spatial information between time frames. The proposed method was tested in a numerical FP-CMR phantom and four patients with suspected CHD.Methods
Digital phantom Fully-sampled FP-CMR data was simulated using the MRXCAT13 phantom and the following parameters: FOV=320x320x80mm3, resolution=2x2mm2, slice thickness=5mm, TS/TR/TE=150/2/1ms, flip angle=15deg, contrast agent dose=0.075 mmol/kg, contrast relaxivity=5.6 L/mmol·s, 6 coils, 32 time frames and population averaged arterial input function (AIF). Six Gaussian noise realizations (CNR=40) were performed for each dataset.In-vivo experiments Rest fully-sampled FP-CMR acquisitions were performed in four patients with suspected CHD using a dual-bolus technique with 0.0075+0.075mmol/kg Gadobutrol (Gadovist; Bayer, Germany). Patients 1&2 were scanned on a 3T Philips Achieva scanner and patients 3&4 were scanned on a 1.5T Philips Ingenia scanner (Philips Healthcare, Netherlands). A saturation-recovery turbo field echo (TFE) sequence was used to acquire one short-axis (SAX) slice in free-breathing using the following parameters: FOV=320x320mm2, resolution=2.8x2.8mm2, slice thickness=10mm, TS/TR/TE= 120/1.96/0.93ms, flip angle=15deg, acquisition window=224-228ms, total acquisition time=1min20s, and contrast agent relaxivity=5.0 L/mmol·s. The AIF was found using a region of interest in the left ventricle. The fully-sampled dynamic images were used to estimate the frame-to-frame translational motion. Then, translational motion correction was performed directly in k-space to generate motion-corrected datasets.
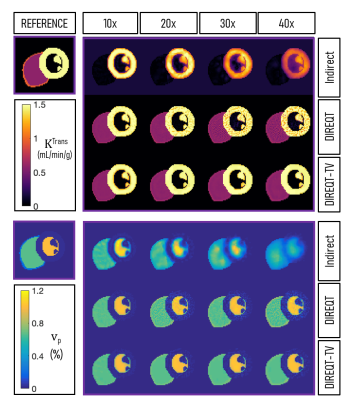
Reconstruction Radial k-t sampling was used to generate 10x, 20x, 30x and 40x undersampled datasets, which were reconstructed using the indirect and DIREQT methods (Fig.1). The DIREQT method directly estimates TK parameter maps from the measured FP-CMR data. This is achieved by solving the following optimization problem: $$$\hat{\mathbf{M}}=\arg\min\limits_{\mathbf{M}} \left\|\mathbf{d}-f\left(\mathbf{M}\right)\right\|_2^2$$$, where $$$\mathbf{M}$$$ are the TK parameters maps (e.g. KTrans and vp of the Patlak model), $$$\mathbf{d}$$$ is the undersampled (k-t)-space data and $$$f$$$ is the forward model (indicated by the small red arrows in Fig.1). A limited memory BFGS quasi-Newton method was used to solve this nonlinear inverse problem. The value of adding spatial total variation regularization to DIREQT (DIREQT-TV) was also tested. Reconstructions were performed using MATLAB (MathWorks, USA) on an Intel i7-86508 @ 1.9 GHz laptop with 32 GB memory.
Results
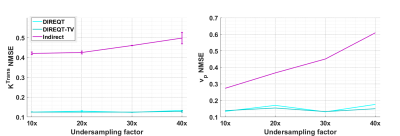
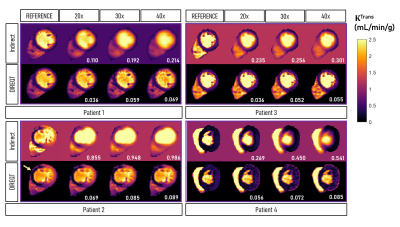
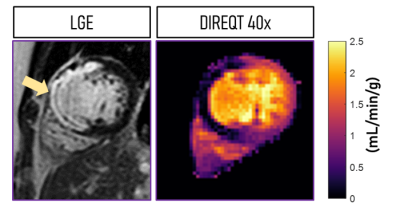
Figure 2 shows the DIREQT, DIREQT-TV and indirect reconstructions obtained from simulated phantom data. DIREQT maps have superior image quality compared to the indirect method maps, particularly at high undersampling rates. In addition, TV regularization helps reduce noise amplification at high accelerations (Fig. 2). The lowest normalized mean square error (NMSE) was achieved with DIREQT (Fig. 3), which indicates a better agreement with the reference.Figure 4 shows the quantitative maps obtained from patient data using the indirect and DIREQT methods. Patient 2 had myocardial infarction and patient 4 hypertrophic cardiomyopathy. Patients 1 and 3 had no abnormalities on CMR. The perfusion defect identified in Patient 2 DIREQT maps corresponds to an area of myocardial scarring, as evidenced by the late gadolinium enhancement (LGE) image (Fig. 5). DIREQT provides high-quality maps even at very high acceleration rates, whereas the indirect method provides images with insufficient diagnostic quality. The time gained, by greatly accelerating acquisitions, could be used to achieve much higher spatial resolution and coverage, and hence, greater diagnostic accuracy. The total reconstruction times for the indirect and DIREQT methods were ∼290s and ∼185s, respectively.
Conclusions
A direct model-based reconstruction approach was proposed to highly accelerate FP-CMR acquisitions. The proposed DIREQT method combines image reconstruction and tracer-kinetic modeling to generate quantitative myocardial perfusion maps directly from the acquired data. DIREQT exploits the redundancies in FP-CMR data, thus enabling high acceleration factors (up to 40x). In future studies, the DIREQT method will be evaluated in a large cohort of patients with suspected CHD using prospective undersampled acquisitions. These studies will also aim to achieve much higher spatial resolution and coverage, and hence, greater diagnostic accuracy. Different TK models and self-navigation strategies (for respiratory motion estimation/correction) will also be explored.Acknowledgements
This work was supported by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z].References
1. Kellman P, Arai AE. Imaging sequences for first pass perfusion - a review. J Cardiovasc Magn Reson. 2007;9(3):525-37.
2. Fair MJ, Gatehouse PD, DiBella EV, Firmin DN. A review of 3D first-pass, whole-heart, myocardial perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2007;9(3):525-37.
3. Villa ADM, Corsinovi L, Ntalas I, et al. Importance of operator training and rest perfusion on the diagnostic accuracy of stress perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2018;20(1):74.
4. Kellman P, Hansen MS, Nielles-Vallespin S, et al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson. 2017;19(1):43.
5. Hsu LY, Jacobs M, Benovoy M, et al. Diagnostic performance of fully automated pixel-wise quantitative myocardial perfusion imaging by cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2018;11(5):697-707.
6. Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med. 2010;64(3):767-76.
7. Vitanis V, Manka R, Giese D, et al. High resolution three-dimensional cardiac perfusion imaging using compartment-based k-t principal component analysis. Magn Reson Med. 2011;65(2):575-87.
8. Lingala SG, Hu Y, DiBella E, Jacob M. Accelerated dynamic MRI exploiting sparsity and low-rank structure: k-t SLR. IEEE Trans Med Imaging. 2011;30(5):1042-54.
9. Chen X, Salerno M, Yang Y, Epstein FH. Motion-compensated compressed sensing for dynamic contrast-enhanced MRI using regional spatiotemporal sparsity and region tracking: block low-rank sparsity with motion-guidance (BLOSM). Magn Reson Med. 2014;72(4):1028-38.
10. Petibon Y, Rakvongthai Y, El Fakhri G, Ouyang J. Direct parametric reconstruction in dynamic PET myocardial perfusion imaging: in vivo studies. Phys Med Biol. 2017;62(9):3539-3565.
11. Guo Y, Lingala SG, Zhu Y, et al. Direct estimation of tracer-kinetic parameter maps from highly undersampled brain dynamic contrast enhanced MRI. Magn Reson Med. 2017;78(4):1566-1578.
12. Dikaios N, Arridge S, Hamy V. Direct parametric reconstruction from undersampled (k,t)-space data in dynamic contrast enhanced MRI. Med Image Anal. 2014;18(7):989-1001.
13. Wissmann L, Santelli C, Segars WP, Kozerke S. MRXCAT: Realistic numerical phantoms for cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2014;16:63.
Figures