1015
Stack-of-Stars Inversion-Recovery MRI for Free-Breathing T1 Mapping and IR-Prepared Fat/Water Separation1Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, New York University School of Medicine, New York, NY, United States, 3MR Applications Development, Siemens Healthcare GmbH, Erlangen, Germany, 4Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 5Gordon Center for Medical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 6Department of Radiology, University of Wisconsin Madison, Madison, WI, United States
Synopsis
This work presents a framework for inversion-recovery (IR)-prepared stack-of-stars imaging and its applications for rapid free-breathing 3D liver MRI. Building upon a previously developed stack-of-stars 3D GRE sequence (RAVE: RAdial Volumetric Encoding), a non-selective 180o IR pulse has been implemented that is periodically played-out to achieve IR preparation (IR-Prepped RAVE). The new sequence allows (1) single-echo acquisition in combination with GRASP-Pro (imProved Golden-angle RAdial Sparse Parallel) reconstruction for free-breathing volumetric T1 mapping of the liver, and (2) multi-echo acquisition in combination with dynamic model-based reconstruction for IR-prepped and contrast-resolved fat/water separation.
INTRODUCTION:
Radial stack-of-stars sampling offers various advantages for body MRI, including inherent robustness to motion, benign/incoherent undersampling behavior that can be synergistically combined with sparsity-based reconstruction, and self-navigation capabilities for additional motion management. Its performance has been demonstrated in gradient-echo (GRE) (1), fast spin-echo (FSE) (2), multi-echo Dixon (3), dynamic contrast-enhanced (DCE) (4), and balanced steady-state free precession (bSSFP) imaging (5). However, the combination of stack-of-stars MRI with magnetization preparation has not been widely explored yet. In this work, we present a framework for inversion-recovery-prepared (IR-prepped) stack-of-stars imaging and demonstrate its feasibility for rapid volumetric T1 mapping and IR-prepped fat/water separation in the liver during free breathing.METHODS:
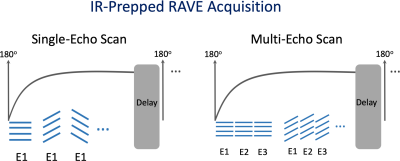
(a) Sequence development: Building upon a previously developed stack-of-stars 3D GRE sequence (RAVE: Radial Volumetric Encoding) (3), an adiabatic non-selective 180o IR pulse has been implemented that is periodically played-out to achieve magnetization preparation. The modified sequence (referred to as IR-Prepped RAVE) allows for both single-echo acquisition (for 3D T1 mapping) and multi-echo acquisition (for IR-prepped fat/water separation). After each IR pulse, a series of radial stacks (referred to as “stack-train”), rotated by the golden angle (111.25o) (6), are acquired until the magnetization reaches steady state, as illustrated in Figure 1. The acquisition is followed by an idle period to ensure full magnetization recovery before the next IR pulse; and golden-angle rotation continues for the following stack-trains to enable flexible data sorting. The IR-prepped acquisition scheme is repeated N times, which can be determined by the user according to specific applications.(b) Data acquisition: The feasibility of IR-Prepped RAVE imaging has been demonstrated for free-breathing 3D T1 mapping of the liver and IR-prepped fat/water separation.
T1 Mapping: Single-echo IR-Prepped RAVE was acquired in a T1-mapping phantom and in one volunteer (female, age=26) on a 3.0T clinical MR scanner (MAGNETOM Skyra, Siemens). Relevant imaging parameters for both scans included: FOV=350x350mm2, in-plane resolution=1.37x1.37 mm2, slice thickness=5mm, flip angle (FA)=5o, number of slices=32 with 75% partial Fourier, TR/TE=2.63/1.23ms, bandwidth=1050Hz/pixel. Each stack-train comprised 56 radial stacks, and the idle period was set to 5 seconds. The IR-prepped acquisition was repeated for 25 times, leading to a total acquisition time of ~3.5 minutes. For comparison, 2D Modified Look-Locker Imaging (MOLLI) was acquired with identical spatial resolution and this was performed under breath hold for the volunteer scan.
Fat/Water Imaging: Three-echo IR-Prepped RAVE was acquired in one volunteer (male, age=34) on the same MR scanner. Imaging parameters were the same as above except for TR (6.02ms) and TEs (1.41/3.01/4.61ms). The IR-prepped acquisition was repeated for 25 times with a total acquisition time of ~5.5 minutes. For comparison, three-echo RAVE imaging (without IR preparation) was performed with similar imaging parameters except for the FA (12o). A total of 600 spokes were acquired and the total acquisition time was ~2 minutes.
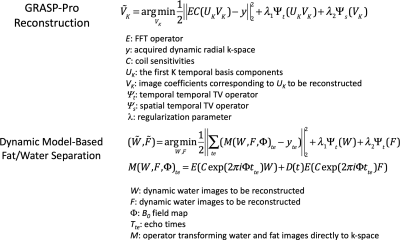
(c) Image reconstruction: GRASP-Pro (imProved Golden-angle RAdial Sparse Parallel) reconstruction (7) was applied to process the single-echo data. As indicated by the cost function 1 in Figure 2, the reconstruction employs a subspace constraint in combination with a temporal total-variation (TV) constraint along the image series and a spatial TV constraint directly on the subspace. The temporal basis used for the subspace generation was estimated from a dictionary generated using Bloch equation with T1 value ranging from 100-3000ms and B1 (to account for flip angle variation) ranging from 0.7 to 1.3. After image reconstruction, a three-parameter T1 recovery model was used to calculate the T1 value on a pixel-by-pixel basis. For IR-prepped multi-echo data, a dynamic model-based reconstruction (3) was employed for fat/water separation at different inversion-recovery (TI) times, with a spatiotemporal TV constraint applied on both the water and fat image series (cost function 2, Figure 2). For reference multi-echo RAVE data (without IR preparation), a static model-based reconstruction for fat/water separation was applied without explicit regularization (3).
RESULTS:
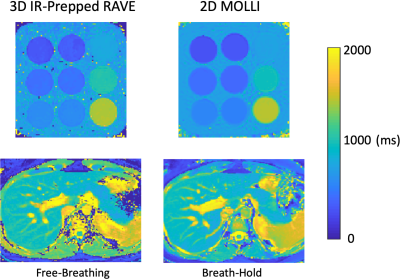
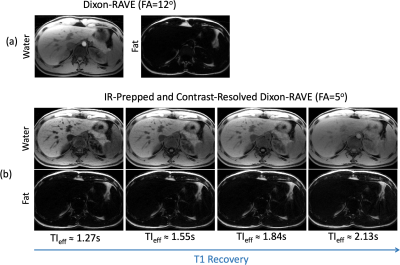
Figure 3 compares T1 maps generated from 3D IR-Prepped RAVE and 2D MOLLI. For both the phantom and volunteer data, T1 values are in good agreement between the two imaging approaches. The T1 value of the liver is around 800-850ms, which is in-line with previous literatures (8). Figure 4 shows additional representative slices of T1 maps obtained from the IR-Prepped RAVE imaging. Figure 5 compares reference multi-echo fat/water images (without IR preparation) to multi-echo IR-Prepped fat/water images at different TI times, where the IR preparation enables contrast-resolved fat/water separation with greatly improved image contrast.DISCUSSION:
This work demonstrates the feasibility and performance of IR-prepped stack-of-stars MRI for volumetric T1 mapping and fat/water separation of the liver during free breathing. Visually accurate T1 maps were obtained in our initial phantom and volunteer test. IR-prepared fat/water imaging provides improved contrast that can potentially be applied for better characterization of liver lesions, especially in patients with fatty-liver disease. Additional experiments in both volunteer and patient studies will be performed in the next step to fully validate the performance of this technique. Moreover, volumetric T1 mapping and fat/water separation could be further combined into a single comprehensive acquisition, and this will be investigated in future works.Acknowledgements
No acknowledgement found.References
1. Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, Babb JS, Kiefer B, Lee VS. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Investigative radiology 2011;46(10):648-653.
2. Benkert T, Mugler JP, 3rd, Rigie DS, Sodickson DK, Chandarana H, Block KT. Hybrid T2 - and T1 -weighted radial acquisition for free-breathing abdominal examination. Magn Reson Med 2018;80(5):1935-1948.
3. Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magn Reson Med 2017;78(2):565-576.
4. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med 2014;72(3):707-717.
5. Liu J, Spincemaille P, Codella NC, Nguyen TD, Prince MR, Wang Y. Respiratory and cardiac self-gated free-breathing cardiac CINE imaging with multiecho 3D hybrid radial SSFP acquisition. Magn Reson Med 2010;63(5):1230-1237.
6. Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging 2007;26(1):68-76.
7. Feng L, Wen Q, Huang C, Tong A, Liu F, Chandarana H. GRASP-Pro: imProving GRASP DCE-MRI through self-calibrating subspace-modeling and contrast phase automation. Magn Reson Med 2020;83(1):94-108.
8. Chen Y, Lee GR, Aandal G, Badve C, Wright KL, Griswold MA, Seiberlich N, Gulani V. Rapid volumetric T1 mapping of the abdomen using three-dimensional through-time spiral GRAPPA. Magn Reson Med 2016;75(4):1457-1465.
Figures