1014
Free-breathing Abdominal Fat Spectroscopy with Multi-Echo Rosette k-space Sampling
Suma Anand1, Adam Michael Bush2, Christopher Michael Sandino3, Shreyas Vasanawala2, and Michael Lustig1
1Electrical Engineering and Computer Sciences, University of California, Berkeley, Berkeley, CA, United States, 2Radiology, Stanford University, Palo Alto, CA, United States, 3Electrical Engineering, Stanford University, Palo Alto, CA, United States
1Electrical Engineering and Computer Sciences, University of California, Berkeley, Berkeley, CA, United States, 2Radiology, Stanford University, Palo Alto, CA, United States, 3Electrical Engineering, Stanford University, Palo Alto, CA, United States
Synopsis
Obesity is a major cause of preventable morbidity and mortality in the US. A growing body of work suggests that triglyceride composition and its spatial distribution play a central role in this epidemic, necessitating the need for better non-invasive fat imaging. We propose a motion-robust acquisition scheme that combines the spatial resolution of MRI and the spectral resolution of MR spectroscopy using 2D multi-echo rosette k-space sampling. We validate the method with an oil phantom and demonstrate its motion robustness with a free-breathing in vivo acquisition.
Introduction
Obesity is strongly linked to non-alcoholic fatty liver disease, diabetes, and cardiovascular disease, and is the major cause of preventable morbidity and mortality in the US. Global fat burden has been implicated in disease, but a growing body of work suggests triglyceride composition and its spatial distribution also play a central role, necessitating the need for better non-invasive fat imaging1.MRI is excellent at quantifying the amount and spatial localization of fat based on the 3.4ppm spectral shift of water and fat hydrogen protons2,3. However, fat actually consists of multiple resonances, but poor spectral resolution and low SNR have limited MRI-based methods for triglyceride discrimination. In contrast, MR spectroscopy is well suited for determining triglyceride subtypes but is plagued by poor spatial resolution, long scan times, and motion sensitivity4. Multi-echo approaches have been used to derive fat spectrum and composition parameters based on multipeak models5,6. Separately, non-Cartesian sampling approaches have been successful at reducing motion related artifacts for in vivo fat fraction determination7. Therefore, by combining these two methods via a multi-echo non-Cartesian acquisition, we propose a high spatial resolution, motion robust, spectroscopic method for triglyceride characterization using a 2D multi-echo rosette trajectory8,9.Methods
We designed a 2D free-breathing imaging protocol using a rosette trajectory8,9 that achieves high spatial and spectral resolutions as well as motion robustness. We validated our method with a phantom containing vials of different fats: olive, coconut, and peanut oil. We compared our method to a free-breathing Cartesian acquisition, which is corrupted by substantial motion artifacts.All imaging was performed on a 3T GE 750W MRI system. A 22-channel head coil and 32-channel cardiac coil were used for phantom and in vivo experiments, respectively. IRB approval and informed consent were obtained for the in vivo scans. For the Cartesian sequence, a gated, unipolar, 8 echo, multi-echo GRE sequence was used with the initial echo time 1.5ms and echo spacing of 2.0ms. Other sequence parameters included FA=25 degrees, TR=15.7 ms, FOV=40 cm, 1.5 mm in-plane resolution, and 8 mm slice thickness. A spoiled gradient recalled, ungated, time-averaged, rosette pulse sequence with FA=15 degrees, TR=17 ms, and rosette shape parameter of 2.2 was used8. The sequence used maximum slew rates and gradient amplitudes of 75 mT/m/s and 40 mT/m, respectively, to reduce eddy current and concomitant gradient related artifacts. A single repetition was rotated by the golden angle, 137.5°, and repeated 800 times. A single scan with ten echoes per TR was used for the in vivo experiments. For the phantom experiments, 11 interleaved acquisitions with five echoes each were taken, each delayed by 0.3ms to achieve a spectral bandwidth of 3.3 kHz.
The data was segmented into echoes (Figure 1), each with a sampling window of 750 samples per segment. Figure 1b shows the echoes for 20 rotations. A local low-rank compressed sensing reconstruction was performed using BART8, reconstructing each echo separately. Images were reconstructed separately from each acquisition and combined to form a time series of 55 echoes. Fat and water images were obtained by a zero-padded, Hann windowed FFT along the time dimension.
Our technique was validated using a phantom consisting of three vials of fats submerged in water: olive, coconut, and peanut oil (Figure 2a). Coconut oil is a saturated fat, while the other two are not; consequently, no peak in the coconut oil spectrum due to olefinic protons (i.e., attached to double-bonded carbons) is expected, but olefinic peaks in the other spectra are expected5. An ROI-based analysis of each vial was performed to test this hypothesis using MATLAB and Python.
Results
Figure 2b shows the median magnitude spectrum across pixels in each ROI. The error bars indicate the standard deviation. The red dashed line and arrow indicate the peak and frequency of interest, respectively. The peak is significantly smaller for the coconut oil ROI than for the other two, which may confirm our hypothesis. No corrections for gradient delay, T2*, or B0 inhomogeneity were performed. Figure 3 compares in vivo motion robustness between a Cartesian free-breathing multi-echo GRE sequence and our rosette multi-echo sequence. No motion correction was performed on either dataset. The images are compared at comparable echo times: 1.8ms, 9.5ms, and 27.5ms for Cartesian and 0.7ms, 10.5ms, and 28.5ms for rosette.The rosette acquisition demonstrates motion robustness while the Cartesian acquisition is heavily corrupted by respiratory motion.Conclusion
We have developed a motion robust method for investigating the spectral properties of fat with a rosette k-space trajectory. Preliminary results demonstrate that we can successfully distinguish fatty acid moieties of common cooking fats as predicted by the literature. Though we are limited by potential artifacts due to gradient delays, T1 recovery and field inhomogeneity, this work indicates that complete spatial resolved, spectral decomposition of fat in vivo is possible using rosette k-space sampling.Acknowledgements
No acknowledgement found.References
- Mumme, K., & Stonehouse, W. (2015). Effects of medium-chain triglycerides on weight loss and body composition: a meta-analysis of randomized controlled trials. Journal of the Academy of Nutrition and Dietetics, 115(2), 249-263.
- Dixon, W. T. (1984). Simple proton spectroscopic imaging. Radiology, 153(1), 189-194.
- Reeder, S. B., et al (2005). Iterative decomposition of water and fat with echo asymmetry and least‐squares estimation (IDEAL): application with fast spin‐echo imaging. Mag. Res. Med., 54(3), 636-644.
- Bucholz, E. K., et al (2008). Multispectral imaging with three‐dimensional rosette trajectories. Mag. Res. Med., 59(3), 581-589.
- Berglund, J., Ahlström, H., & Kullberg, J. (2012). Model‐based mapping of fat unsaturation and chain length by chemical shift imaging—phantom validation and in vivo feasibility. Mag. Res. Med, 68(6), 1815-1827.
- Eggers, H., & Börnert, P. (2014). Chemical shift encoding‐based water–fat separation methods. Journal of Magnetic Resonance Imaging, 40(2), 251-268.
- Armstrong, T., et al (2018). Free‐breathing liver fat quantification using a multiecho 3 D stack‐of‐radial technique. Mag. Res. Med., 79(1), 370-382.
- Noll, D. C. (1997). Multishot rosette trajectories for spectrally selective MR imaging. IEEE Trans. Med. Imaging, 16(4), 372-377.
- Bush, A.M., et al (2019). Multi-Echo Flow-encoded Rosette (MELROSE) enables velocity and T2* assessment of both extravascular tissue and intravascular blood for motion robust, quantitative cardiovascular blood flow and oxygenation mapping. Proc. Intl. Soc. Mag. Reson. Med., 27.
Figures
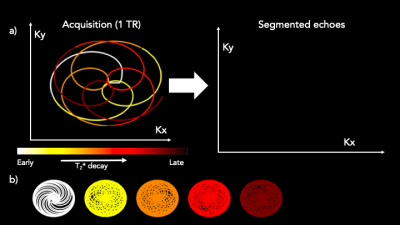
The acquisition for a multi-echo rosette trajectory is shown. In each TR, a “flower” with five to ten echoes is acquired, then rotated by the golden angle. The data is segmented into echoes, shown for one TR in the animation in Figure 1a, and shown for multiple rotations in Figure 1b.
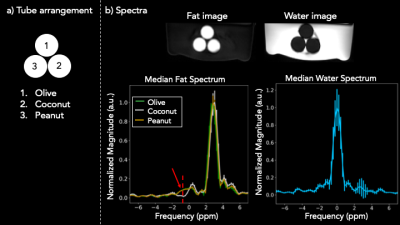
2a: The arrangements of the three oil tubes submerged in water - olive, coconut and peanut oil - is shown. 2b: An ROI-based analysis was performed for each of the oil tubes. The water and fat images were obtained by a zero-padded, Hann windowed FFT across the echo time dimension. The median across each ROI is shown with standard deviation as error bars. The red peak indicates the hypothesized peak of the olefinic resonance of coconut oil. Since coconut oil is saturated, but the other two are not, the peak of its olefinic resonance is much lower.
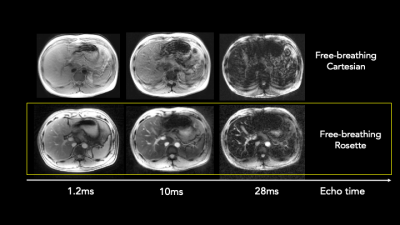
We demonstrate the motion robustness of our acquisition (bottom) by comparing it to a free-breathing Cartesian acquisition (top) at comparable echo times. The number shown is the average of the two echo times. Each image is windowed separately to emphasize motion-related artifacts. The Cartesian acquisition shows severe motion artifacts, while our rosette acquisition does not.