0997
A Model-Based Variational Neural Network for Accelerated and Respiratory Motion-resolved 4D Cartesian Cardiac MRI1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
Long scan times and susceptibility to respiratory motion are major challenges in free-breathing 3D cardiac MRI. Respiratory-resolved 4D approaches deal with motion by assigning data to different respiratory bins and exploiting motion redundancies during reconstruction. However, for accelerated acquisitions this leads to highly undersampled respiratory bins, affecting image quality. Here we propose a novel unrolled VNN that reconstructs undersampled 4D cardiac MRI by exploiting motion redundancies and by using conjugate gradient to enforce data-consistency within every stage of the VNN, providing generalization of the network to the unpredictable sampling of each bin due to subject-specific respiratory motion.
INTRODUCTION
Long scan times and susceptibility to respiratory motion are major challenges in free-breathing 3D whole-heart MRI. Recently, a Variational Neural Network1 (VNN) reconstruction framework has been proposed to accelerate respiratory motion-compensated 3D whole-hearth MRI2. However, this approach only corrects for 2D translational motion and image quality can be affected by residual non-rigid motion. To account for 3D non-rigid motion, the acquired data can be divided into multiple respiratory phases (or bins) based on the 2D iNAV head-feet displacement to generate 4D respiratory-resolved images3,4. However, unrolled VNN reconstruction methods are not robust to large differences in sampling trajectories encountered in each bin due to subject-specific respiratory motion (Fig 1). Here, we propose an optimized unrolled VNN network approach for respiratory motion-resolved undersampled 3D whole-heart MRI. The novel network architecture enforces data-consistency by proposing to include a model-based conjugate-gradient (CG) algorithm within every stage of the VNN. Additionally, the weights are shared for every stage5. This unrolled network architecture, with shared weights, exploits temporal redundancies and offers higher robustness to variations in undersampling artefacts, compared to methods that rely on residual gradients steps to enforce data consistency, as in the conventional VNN1 architecture. The proposed unrolled reconstruction network (CG-VNN) was compared against iterative-SENSE6 and conventional VNN1 reconstructions for six healthy subjects and six patients with congenital cardiovascular disease (CHD), for an acquisition acceleration factor of 5x and 5 respiratory bins.METHODS
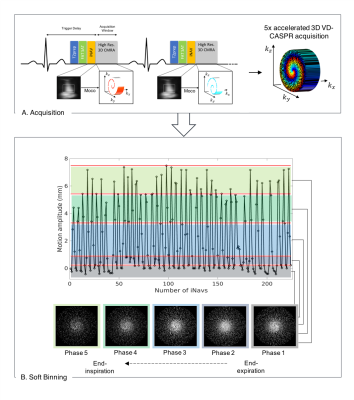
Imaging: Free-breathing ECG-triggered whole-heart 3D MR bSSFP data were acquired on a 1.5T scanner (Siemens Magnetom Aera). Two different imaging protocols - coronary MR angiography (CMRA) and Magnetization Transfer-Inversion Recovery (MTC-BOOST7) - were acquired in healthy subjects and patients with CHD, respectively. Sixteen healthy subjects underwent CMRA with the following parameters: 1.2mm3 isotropic resolution, 5-fold undersampling and fully-sampled (for VNN training), FOV 320x320x86-115mm3, T2-preparation duration 40ms, TE/TR 1.6/3.7ms, FA 90°, bandwidth 890Hz/pixel. Six CHD patients underwent MTC-BOOST7 with the following parameters: resolution 1.4x1.4x2.8mm3 (reconstructed to 1.4mm3 isotropic), of 5-fold undersampling, FOV 320×320×90–120mm3, TE/TR 1.5/3.2ms, FA 90°, bandwidth 890Hz/pixel, MT-preparation: 15 pulses, bandwidth‐time‐product 1.92, FA 800°, pulse duration 20.48ms, off‐resonance frequency offset 3000Hz. Ten healthy subjects (CMRA) were considered for training, whereas 6 healthy subjects (CMRA) and 6 CHD patients (MTC-BOOST) were used for evaluation.Acquisition and respiratory binning: Undersampled acquisitions are performed with a variable-density Cartesian acquisition with spiral-like profile order8,9 (Fig. 1A). A 2D image-navigator (iNAVs) enables beat-to-beat translational respiratory motion estimation and 100% respiratory scan efficiency10. Undersampled acquired data is sorted into 5 respiratory bins, using the foot-head motion estimated from iNAVs and soft-binning3 (Fig 1B). Additionally, 2D beat-to-beat intra-bin translational motion correction is performed. Representative undersampled soft-binned trajectories are presented in Fig. 1B.
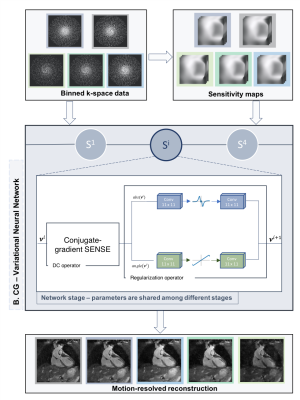
CG-VNN Architecture: Undersampled k-space data for the 5 different respiratory bins, coil sensitivity maps and undersampled soft-binned trajectories are provided as input to the proposed CG-VNN. In each of the 4 stages of the network, the initial reconstruction is updated according to the joint variational scheme in Fig. 2. A first path applies a CG SENSE operator to the undersampled k-space data, accounting for coil-sensitivities and the soft-binned trajectories. The second path is a regularization operator that jointly applies 11x11 filter kernels and corresponding activation functions to the 5 respiratory bins. Two different set of filter kernels and corresponding activation functions are applied to the magnitude and phase of the 5 complexed-valued respiratory bins. In this formulation CG-VNN, all parameters are shared among the stages of the unrolled network architecture.
CG-VNN Training: The network was trained on 2D complex-valued images obtained from retrospectively undersampled 5-fold CMRA data of 10 healthy subjects (2400 images). Training is performed applying 5-fold spiral-like undersampled trajectories, not accounting for difference in trajectories due to subject-specific breathing patterns. During the training procedure, the output of the CG-VNN is compared to the corresponding fully sampled reference images.
RESULTS
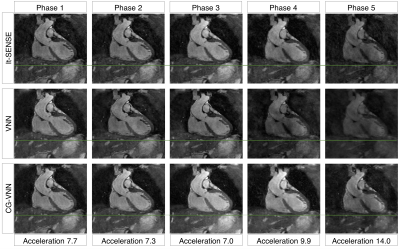
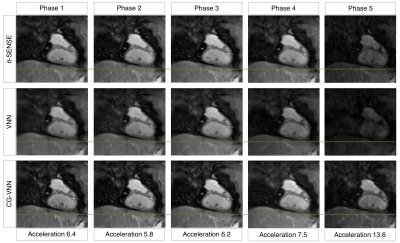
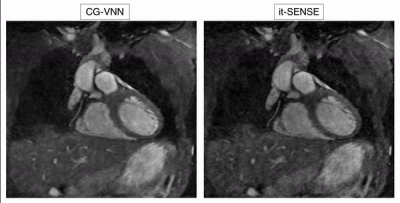
The average scan time was ~4min (CMRA) and ~3min (MTC-BOOST) with 100% respiratory scan efficiency. The average reconstruction time for the 5 respiratory bins was ~15 mins for it-SENSE and ~34s for CG-VNN reconstructions. Respiratory-resolved images obtained with it-SENSE6, conventional VNN1 and the proposed CG-VNN are shown in Fig. 3 for a representative healthy subject and in Fig. 4 for a representative CHD patient. Aliasing artefacts, not present in the CG-VNN reconstructions, are observed for it-SENSE6 and for the conventional VNN1 reconstruction, in particular for end-inspiratory respiratory phases (4 and 5) that present the largest displacement of the heart. Most importantly, the CG-VNN does not result in differences in contrast among different respiratory phases that are present in the conventional VNN1 motion-resolved images. In Fig. 5 motion-resolved CMRA images for a healthy volunteer are presented, showing the displacement of a portion of right coronary artery during respiration.DISCUSSION
We proposed a model-based CG-VNN network framework that reconstructs undersampled 4D (3D respiratory-resolved) cardiac MRI by exploiting motion redundancies and providing generalization of the network to the unpredictable sampling of each bin due to subject-specific respiratory motion. Experimental results in healthy subjects and patients with CHD show that the proposed approach outperforms iterative SENSE and conventional VNN reconstruction. Furthermore, it results in extremely fast reconstruction time of 34s, offering easy integration into clinical workflow.Acknowledgements
This work was supported by EPSRC (EP/P001009, EP/P007619, EP/P032311/1) and Wellcome EPSRC Centre for Medical Engineering (NS/A000049/1).References
1. Hammernik K, et al. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med. 2018;79(6):3055-3071.
2. Fuin N, et al. A Variational Neural Network for Accelerating Free-breathing Whole-Heart Coronary MR Angiography. ISMRM 2019, p 0478, Montreal, Canada.
3. Correia T, et al. Optimized respiratory-resolved motion-compensated 3D Cartesian coronary MR angiography. Magn Reson Med. 2018;80(6):2618-2629.
4. Piccini D, et al. Four-Dimensional Respiratory Motion-Resolved Whole Heart Coronary MR Angiography. Magn Reson Med. 2018;77:1473-1484.
5. Aggarwal K, et al. MoDL: Model Based Deep Learning Architecture for Inverse Problems. IEEE Trans Med Imag. 2019;38:394-405.
6. Pruessmann KP, et al. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001;46:638 – 651.
7. Ginami G et al. Non‐contrast enhanced simultaneous 3D whole‐heart bright‐blood pulmonary veins visualization and black‐blood quantification of atrial wall thickness. Magn Reson Med. 2019;81(2):1066–1079.
8. Prieto C, et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J Magn Res Imag. 2015;41(3):738-746.
9. Bustin A, et al. Five‐minute whole‐heart coronary MRA with sub‐millimeter isotropic resolution, 100% respiratory scan efficiency, and 3D‐PROST reconstruction. Magn Reson Med. 2019;81:102-115.
10. Henningsson M, et al. Prospective respiratory motion correction for coronary MR angiography using a 2D image navigator. Magn Reson Med. 2013;69(2):486- 494.
Figures