0978
Distortion-free, submillimeter-isotropic-resolution diffusion MRI with gSlider BUDA-EPI and multi-coil dynamic B0 shimming1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 2Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Department of Radiology, Faculty of Medicine, Siriraj Hospital, Mahidol University, Thailand
Synopsis
Diffusion magnetic resonance imaging (dMRI) is a highly sensitive imaging modality, but is limited in spatial resolution and signal-to-noise ratio (SNR). In this work, we combine an SNR-efficient acquisition and model-based reconstruction strategies with newly-available hardware instrumentation to achieve distortion-free in-vivo dMRI at 600-860 µm isotropic voxel size with high fidelity and sensitivity on a clinical 3T scanner. At this resolution, it is possible to accurately probe the microstructure of different cortical layers in the human brain.
Introduction
Submillimeter-isotropic-resolution dMRI has shown great potential for characterizing gray-matter1,2. However, the current resolution of in-vivo dMRI (at 1.2-2.0mm-isotropic-resolution) is too low to probe the microstructure in cerebral cortex, which causes undesirable partial volume effects. The key challenges of submillimeter in-vivo dMRI are the decreased SNR, physiological noise-induced phase variations and image distortions from B0-inhomogeneity and eddy-current. To address these issues, we first propose Blip Up-Down Acquisition (BUDA) and joint parallel-imaging reconstruction to achieve distortion-free EPI. Second, we combine BUDA with a self-navigated RF-encoded multi-slab acquisition, termed “Generalized Slice Dithered Enhanced Resolution” (gSlider)3,4, to enable high-isotropic-resolution dMRI with high-fidelity and SNR-efficiency. Finally, we minimize g-factor in gSlider-BUDA, by incorporating slab-by-slab dynamic shimming with a 32-channel AC/DC coil5 into the acquisition to reduce B0-inhomogeneity. This enabled high-quality distortion-free whole-brain 600μm dMRI on a 3T scanner.Methods
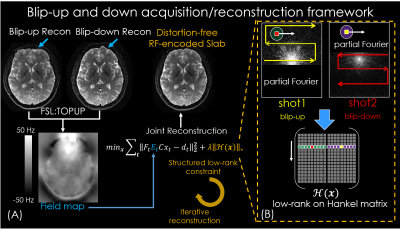
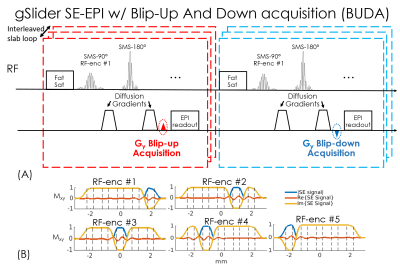
In BUDA-EPI (Fig.1), two EPI-shots sample complementary subsets of k-space, one with a positive ky traversal (blip-up) and the other with a negative traversal (blip-down), to create opposing distortions (Fig.1(B)). As shown in Fig.1(A), the B0 and eddy-current can be extracted from this acquisition pair via FSL ‘TOPUP’6. This information is then incorporated into a joint-reconstruction with a structured low-rank constraint across the two shots to obtain one distortion-free image. Compared to hybrid-space SENSE7, BUDA-EPI obviates the need for direct phase estimation and achieves improved reconstruction at high accelerations. Additionally, a partial Fourier acquisition where EPI-shots sample complementary k-space sections are incorporated directly into the low-rank reconstruction, so a short-TE can be attained to minimize T2-related signal-losses.We combine BUDA-EPI with gSlider, where both blip-up and blip-down shots are acquired in an interleaved fashion (Fig.2(A)). Five consecutive RF-encoding pulses (Fig.2(B)) are used for a total of 10 EPI-shots per slab across blip-up and -down encodings. The RF-excitations are also combined with blipped-CAIPI8 (MB=2) to acquire 10 simultaneous slices per EPI-shot. BUDA reconstruction is first performed for each RF-encoding, followed by gSlider reconstruction4 to obtain high slice-resolution data.
Acquisitions:
(i)To validate gSlider-BUDA, whole-brain 860μm-data were acquired: FOV:220×220×120mm3, TR/TE=3500/68ms. Two-shots were collected at MB=2×Rinplane=4 and partial Fourier (p.f.)6/8. Sixty-four diffusion-directions with b=1000 s/mm2 were acquired.
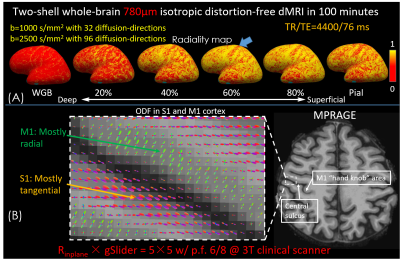
(ii)To investigate the microstructure of gray-matter, whole-brain 780μm-data with two shells were acquired: FOV:220×220×117mm3, TR/TE=4400/76ms. Thirty-two diffusion-directions with b=1000s/mm2 and ninety-six diffusion-directions with b=2500s/mm2 were acquired. To reduce TE and T2* blurring, we used Rinplane=5 with p.f.=6/8 for each shot (resolution point-spread-function ~1.12x for Rinplane=5 at effective-echo-spacing=0.196ms, assuming T2*/T2=30/60ms). 3D-MPRAGE was acquired for surface-based analysis.
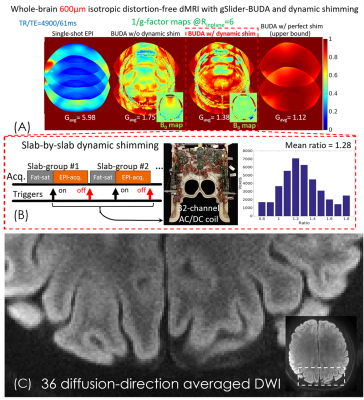
(iii) We further push the spatial-resolution to 600μm isotropic using Rinplane=6 per shot to reduce T2* blurring (point-spread-function ~1.19x) and p.f.=5/8 to reduce the TE to 61ms. To reduce g-factor in the joint distortion-corrected BUDA-reconstruction, AC/DC-coil was utilized for slab-by-slab shimming to reduce B0-inhomogeneity and hence the level of distortion differences between the blip-up and-down data. With dynamic-shimming, 36 diffusion-directions with b=1000s/mm2 and 3 averages were acquired, with FOV:220×220×120mm3, for a total acquisition time of 90 minutes. All in-vivo measurements were performed on a 3T Siemens Prisma scanner.
Post-processing:
Colored-FA maps were generated using FSL6. For cortical depth analysis, the intermediate surfaces between white and pial surfaces were generated by FreeSurfer9 from the MPRAGE data. Then the diffusion principal eigenvectors were aligned to the MPRAGE data10 and projected to intermediate surfaces to calculate the radiality2. For the multi-shell data, ODF was estimated utilizing multi-tissue-multi-shell CSD method11.
Results
Figure3 shows the results of 860μm-data. As white arrows indicate in Fig.3(A), the individual blip-up and -down reconstructions show significant distortions. Compared to the hybrid-space SENSE, the residual artifacts are eliminated in BUDA. Figure3(B) shows the high-fidelity whole-brain diffusion-weighted images (DWI), colored-FA maps and averaged DWIs in three orthogonal views, demonstrating closely matched volumes compared to the reference.Figure4(A) shows the radiality map at the different surfaces on the inflated brain surface. Radiality is low at the white-gray boundary, higher at the middle cortical depths and lower at the pial surface with the low radiality of somatosensory cortex (S1) (indicated by blue arrow) at all cortical depths which reveals the tangential fibers in the S1 area. Figure4(B) shows the ODF in S1 and primary motor cortex (M1). Consistent with previous studies1,2, we find a primarily radial orientation in M1 and tangential fibers to the local cortical surface orientation in S1.
Figure5(A) shows the 1/g-factor maps of single-shot EPI, gSlider-BUDA without and with dynamic shim, and with perfect shim (no B0-inhomogeneity) at Rinplane=6. Compared to the single-shot EPI, BUDA significantly reduces the g-factor penalty at the cost of large g-factor in severe B0-inhomogeneity areas. With dynamic shimming, B0-variation is reduced by>50%, which results in 28% g-factor improvement (Fig.5(B)), approaching performance of the idealized perfect-shimming case (effectively Rinplane=3). Figure 5(C) shows the zoom-in view of a slice of averaged DWI, displaying the high-resolution capability of the 600µm-data.
Discussion and conclusion
In this work, we developed gSlider-BUDA with dynamic shimming to achieve rapid distortion-free submillimeter-isotropic-resolution dMRI. In comparison to standard dMRI, the proposed method has provided improved g-factor and image-fidelity, which is beneficial for co-registration of MPRAGE and research on cortical analysis. This will push in-vivo dMRI toward the submillimeter-scale of cortical layers to transfer new insights from post-mortem imaging to in-vivo imaging.Acknowledgements
This work was supported in part by NIH research grants: NIH R01EB019437, R01EB020613, R01 MH116173, U01EB026996 and U01EB025162References
1. Leuze CWU, Anwander A, Bazin PL, et al. Layer-specific intracortical connectivity revealed with diffusion MRI. Cereb. Cortex 2014;24:328–339.
2. McNab JA, Polimeni JR, Wang R, et al. Surface-based analysis of diffusion orientation for identifying architectonic domains in the in vivo human cortex. Neuroimage 2013;69:87–100.
3. Setsompop K, Fan Q, Stockmann J, et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magn. Reson. Med. 2018;79:141–151.
4. Liao C, Stockmann J, Tian Q, et al. High‐fidelity, high‐isotropic‐resolution diffusion imaging through gSlider acquisition with and T 1 corrections and integrated ΔB0/Rx shim array. Magn. Reson. Med. 2019;83:56–67.
5. Stockmann JP, Witzel T, Keil B, et al. A 32-channel combined RF and B0shim array for 3T brain imaging. Magn. Reson. Med. 2016;75:441–451.
6. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. Fsl. Neuroimage 2012;62:782–790.
7. Zahneisen B, Aksoy M, Maclaren J, Wuerslin C, Bammer R. Extended hybrid-space SENSE for EPI: Off-resonance and eddy current corrected joint interleaved blip-up/down reconstruction. Neuroimage 2017;153:97–108.
8. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med. 2012;67:1210–1224.
9. Fischl B. FreeSurfer. Neuroimage 2012;62:774–781.
10. Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009;48:63–72.
11. Jeurissen B, Tournier J-D, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 2014;103:411–426.
Figures