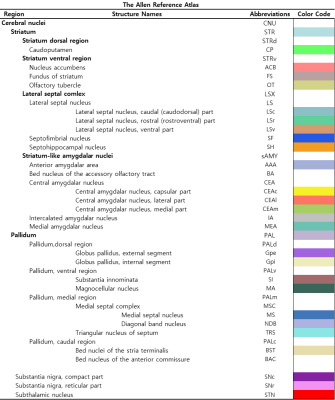0977
Semi-automated tractography analysis using a Allen mouse brain atlas : comparing DTI acquisition between NEX and SNR1Gachon Advanced Institute for Health Sciences & Technology, Gachon university, Incheon, Korea, Republic of, 2Gachon university, Incheon, Korea, Republic of
Synopsis
Although tractography research was focused primarily on the human brain, tractography was integrated into animal models to benefit from various preclinical experiments. Accurate segmentation is required for proper connectome of animal models. The Allen mouse brain atlas can provide accurate coordinates and segmentation information to the mouse brain, but it is difficult to use because it is not MRI data. In this study, we use the ABA to accurately segment the mouse brain and examine tractography. In addition, various NEX are used to determine the changes in tractography caused by an increase in the SNR of the DTI.
Introduction
A comprehensive network of neurons can be mapped using probabilistic c tractography on diffusion tensor images (DTI), reconstructing white matter pathway of the brain1. While studies using tractography mainly focused on connectome mapping of human brains, studies have incorporated tractography in animal models for documenting effects of various pre-clinical trials. However, there has been a lack of comprehensive studies on mouse brain connectivity as well as difficulty in establishing a consensus regarding mouse neural networks2. For proper connectome mapping of animal models, it is necessary that regions of interests are segmented accurately but has been difficult due to slow updates and less interest regarding atlas-based neuroinformatics of animal models3. The widely utilized Allen Mouse Brain Atlas is capable of providing accurate coordinates and segmentations of mouse brain structures imperative for tractography4. To provide reference for mouse connectome mapping, this study explores a method for semi-automatic segmentation and tractography analysis of mouse brain with Atlas Normalization Toolbox using elastix (ANTx) and FSL's PROBTRACKX, using Allen Mouse Brain Atlas as basis. This study also uses a range of numbers of excitation (NEX) to compare the difference in connectivity due to increase in signal-to-noise ratio (SNR) of DTI images.Method
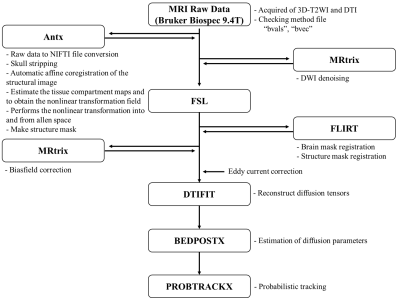
This study was conducted on a 9.4 T Bruker BioSpec horizontal bore, dedicated animal scanner (Bruker Biospin, Ettlingen, Germany), equipped with a gradient system of (440mT/m). For RF excitation a quadrature volume resonator (inner diameter (116mm); Bruker Biospin) was used. For signal reception, a quadrature mouse brain surface coil (Bruker Biospin) was applied. MRI data was acquired using Paravision 5.1 software. All experiments were performed on C57BL/6J mouse. Mouse transcardially were perfused and fixed with 4% paraformaldehyde and 0.1% Magnevist® in phosphate buffer (PB). Brains were extracted and incubated in 0.1% Magnevist/phosphate buffer for 4 days, placed in Fomblin and imaged. The pulse sequence used for this acquisition was 3D TurboRARE T2 (Spin echo sequence with a repetition time = 1800 ms, echo time = 33.6 ms, flip angle = 90°, Bandwidth = 100kHz, field of view = 1.2 × 1.2 × 15.6 cm, matrix = 240 × 240 × 156, resolution = 50 × 50 × 100 µm, 1 averages and resulting in a total acquisition time of 1h 43m) and 2D EPI-Diffusion tensor (Spin echo sequence with a repetition time = 3000 ms, echo time = 30 ms, flip angle = 90°, bandwidth = 170kHz, b-value = 3003 s/mm², diffusion gradient pulse duration (δ) = 4.5 ms, diffusion gradient separation (Δ) = 10.6 ms, field of view = 1.8 × 1.8 cm, slice thickness = 0.2 mm, matrix = 90 × 90, slice = 40, resolution = 200 x 200 x 200 µm. For same sample NEX = 7 (1, 2, 4, 8, 16, 32, 64) data set. Diffusion tensor image data were denoised and biasfield corrected using MRtrix3. We acquired brain extracted images from whole-head input 3D T2 image data and created masks based on Allen Mouse Brain Atlas anatomical regions using ANTx5,6,7. Subsequently, we registered the basal ganglia masks obtained using ANTx to our native mouse brain using FSL's FLIRT tool8. We preformed eddy correction using FSL and then acquired fiber reconstruction data using FSL's BEDPOSTX9 and probabilistic tractography data using FSL's PROBTRACKX10. The image processing pipeline is shown in Figure 1.Result
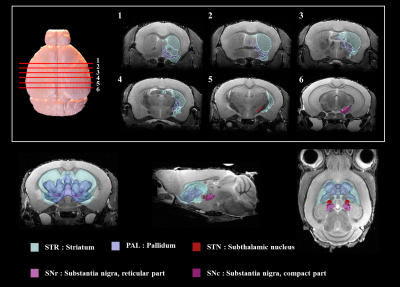
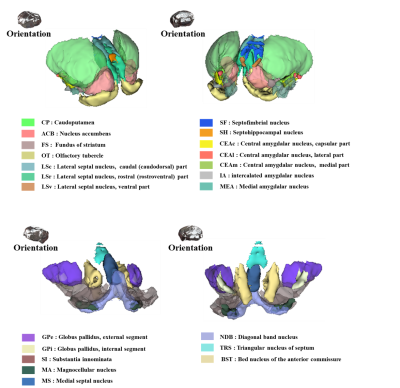
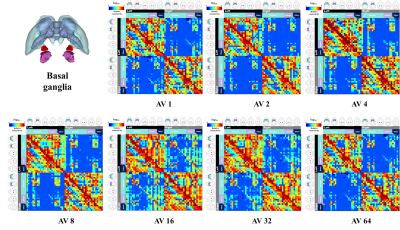
Figure 2 shows our segmentations of the basal ganglia. In addition, we visualized our segmentations on top of structural images to validate our results (top). 3D visualization was done by partitioning the striatum (STR), pallidum (PAL), substantia naigra, reticura (SNr), substantia naigra, compact part (SNc) and subthalamic nucleus (STN) structures. (bottom). Table 1 shows the name and abbreviation of the regions segmented in our study. For each structure, the name and abbreviation were acquired from Allen reference atlas4. The name and color code of the Workplace template used to create this segmentation are shown in Figure 2 and Figure 3. Figure 3 shows visualization was done by partitioning the striatal and pallidal structures in relation to anatomical landmarks. Probabilistic tractography between basal ganglia structures are represented in 7 connectivity matrices, each with different NEX values, to compare connectivity and SNR between each basal ganglia structure as shown in Figure 4.Discussion and conclusion
We performed segmentation and visualization using Allen Mouse Brain atlas anatomical regions. 52 striatal, pallidal and basal ganglia-related structures were segmented in our study. We compared the connectivity matrices generated from diffusion-weighted images with increasing NEX from 1 to 64. We observed an increase in interconnectivity with increasing SNR, as represented by the 7 NEX connectivity matrices. Interestingly, the change in connectivity intensity from 1 NEX to 64 NEX of DTI varied depending on the range of NEX. In particular, the matrices generated with NEX values 1 to 32 showed a linear relationship with SNR, which were consistent with previous studies. However, matrices generated with NEX value of 64 showed no increase in SNR, possibly due to motion and pulsation artifacts contributing to image degradation with additional NEX despite motion compensation being applied to all data sets11. In this study, we were able to utilize semi-automatic segmentation to study anatomical and functional information of the mouse brain.Acknowledgements
This research was supported by Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science andICT (NRF-2017M3C7A1044367).References
[1] Maier-Hein KH, Neher PF, Houde JC, et al. The challenge of mapping the human connectome based on diffusion tractography. Nature communications, 2017, 8.1: 1349.
[2] Oh SW, Harris JA, Ng L, et al. A mesoscale connectome of the mouse brain. Nature, 2014, 508.7495: 207.
[3] Pallast N, Diedenhofen M, Blaschke S, et al. Processing pipeline for Atlas-based Imaging Data Analysis of structural and functional mouse brain MRI (AIDAmri). Frontiers in neuroinformatics, 2019, 13: 42.
[4] Dong HW. The Allen reference atlas: A digital color brain atlas of the C57Bl/6J male mouse. John Wiley & Sons Inc, 2008.
[5] Koch S, Mueller S, Foddis M, et al. Atlas registration for edema-corrected MRI lesion volume in mouse stroke models. Journal of Cerebral Blood Flow & Metabolism, 2019, 39.2: 313-323.
[6] Huebner NS, Mechling AE, Lee HL, et al. The connectomics of brain demyelination: functional and structural patterns in the cuprizone mouse model. Neuroimage, 2017, 146: 1-18.
[7] Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature, 2007, 445.7124: 168.
[8] Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis, 2001, 5.2: 143-156.
[9] Jbabdi S, Sotiropoulos SN, et al. Model‐based analysis of multishell diffusion MR data for tractography: How to get over fitting problems. Magnetic resonance in medicine, 2012, 68.6: 1846-1855.
[10] Behrens TEJ, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 2003, 50.5: 1077-1088.
[11] Soman S, Holdsworth SJ, Skare S, et al. Effect of number of acquisitions in diffusion tensor imaging of the pediatric brain: optimizing scan time and diagnostic experience. Journal of Neuroimaging, 2015, 25.2: 296-302.
Figures