0974
Simultaneous distortion and motion correction in abdominal DW-MRI using dual echo EPI and slice-to-volume registration1Radiology, Boston Children's Hospital, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Radiology, Brigham and Women's Hospital, Boston, MA, United States, 4Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Diffusion-weighted MRI (DW-MRI) has been increasingly used in abdominal applications. However, unavoidable respiratory motion, as well as B0 field inhomogeneities reduce the accuracy of the quantitative parameters and hinders clinical applicability. In this work, we present a dual echo EPI DW-MRI and slice-to-volume registration method to jointly correct for geometric distortion and motion of the kidneys. The results show that our method effectively reduced geometric distortions, improved alignment of the DW-MR volumes and increased the precision of the estimated quantitative parameters.
Introduction
Diffusion-weighted MRI (DW-MRI) has been increasingly used in abdominal applications, especially in pediatric populations, because it is free of contrast agent and ionizing radiation. It has been successfully applied to kidneys, bowel, spleen and liver. DW-MR images are acquired at multiple b-values and directions and quantitative parameters are estimated by fitting a signal decay model such as a diffusion tensor (DTI) or intravoxel incoherent motion (IVIM) model [5]. However, physiological motion (such as respiration, bowel motility) and large geometric distortions due to inhomogeneities in the B0 field during EPI acquisition degrade the image quality and reduce the accuracy of quantitative parameters for diagnosis and assessing response-to-therapy and impede the clinical applicability. In the absence of motion, the geometric distortions can be corrected by first estimating the distortion field from two EPI volumes acquired with opposite phase encoding sweep direction (Left (L)->Right(R) and R->L) and then using the estimated field to correct the entire set of volumes [6]. In the presence of unavoidable motion in abdominal imaging, changes in position of the organs create a different distortion field for each acquisition and this method can no longer be used reliably. Moreover, due to the displacement of the underlying tissue, motion also deteriorates the estimation of quantitative parameters. We introduce a method for simultaneous geometric distortion (Di) and motion compensation (MoCo) for DiMoCo-DW-MRI of the abdomen [7]. Our method employs a dual-echo readout after each excitation, to obtain two EPI images with opposite phase encoding direction with only 30-50 ms between each echo, which effectively freezees the motion in between. A field map derived from each readout is then used to correct distortions in each slice. We then employ a slice-to-volume registration technique [3] to compensate for motion before DW-MRI model fitting and parameter estimation.Methods
We implemented a dual-echo EPI sequence and acquired coronal abdominal DW-MR images from 4 healthy subjects (ages 29-38, 3 females). The sequence consisted on 168 DW-MR volumes (10 b-values and 17 directions) acquired on a 3T Siemens Prisma scanner with TE1/TE2/TR 72ms/108ms/7000ms, 18 coronal slices, voxel size= 2.81x2.81x4 mm, b-values 0, 10, 30, 50, 80,120, 200, 400, 600 and 800 s/mm2). We also acquired a T2 HASTE volume as a reference structural image. In the Di step, we used each pair of R->L/L->R images in TOPUP to estimate the distortion field of each slice [8]. Afterwards, we used the estimated distortion field to create a distortion corrected image. In the MoCo step, we used a slice-to-volume registration method [9] which estimates a transformation to align each slice to a reference volume and uses a Kalman filter for improving the estimated transformations of consecutively acquired slices. We applied this method to each kidney separately, after applying a rectangular mask. Finally, we fitted an IVIM model to the DW-MRI volumes. We evaluate our DiMoCo method based on the improvements in the quality of the images and in the precision of the IVIM model parameters. To evaluate the distortion correction method, we compared the resulting DW-MR volumes against the original R->L volumes (i.e. uncorrected) and reference T2 HASTE volumes. For the MoCo, we compare the change in position of the original R->L volumes versus the corrected volume. Finally, for the IVIM fit, we numerically evaluate the improvement in the precision parameters by computing their coefficient of variation (CV) over 100 wild-bootstrap repetitions [10].Results
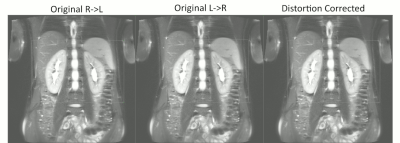
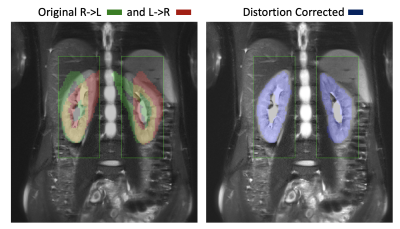
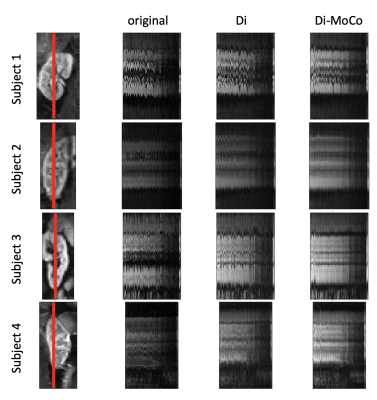
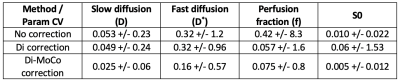
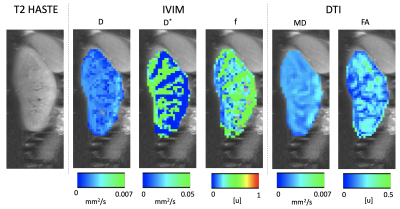
Figures 1 and 2 compare the reference HASTE image against the original R->L, L->R images, as well as the distortion corrected R->L image. The original R->L and L->R images present large geometric distortions in the direction of the phase encoding (left to right) that warp the kidneys, particularly the upper half. After distortion correction, the kidneys are in better alignment with the reference image. We illustrate the motion of the kidneys in time over 168 DW-MR images in Figure 3. A line of voxels (indicated by the red line) for each kidney is plotted across all DW-MR images for the original L->R image (without Di-correction) and Di-corrected image and DiMo-Corrected image. Before Di-Mo-Correction, the plots show oscillatory motion of the kidneys due to breathing as well as increased artifacts due to distortion. After Di-correction, the geometric distortion is reduced and after Di-MoCo, the oscillations due to breathing motion is reduced and the kidneys are aligned in all DW-MR images. The precision of the IVIM model parameters increased when using the Di-MoCo technique (Table 1). The CV of the perfusion fraction reduced from 0.42 +/- 8.3 to 0.075 +/-0.8 when applying the proposed Di-MoCo-DW-MRI. Finally, we show an example map of the estimated IVIM and DTI parameters in Figure 4.Conclusions
We presented a method for simultaneous geometric distortion (Di) and motion compensation (MoCo) for DiMoCo-DW-MRI of the abdomen. Our method corrects for the B0 field inhomogeneity related geometric distortions and the misalignments due to breathing motion. Our results indicate that the proposed method successfully corrected for distortion and compensated the motion of the kidneys. Consequently, the image quality and the precision of the estimated IVIM model parameters improved with a reduction of the CV with the DiMoCo-DW-MRI technique.Acknowledgements
This work was supported partially by the Boston Children's Hospital Translational Research Program Pilot Grant 2018, Society of Pediatric Radiology Multi-center Research Grant 2019, Crohn’s and Colitis Foundation of America’s (CCFA) Career Development Award and AGA-Boston Scientific Technology and Innovation Award 2018 and by NIDDK of the National Institutes of Health under award number R01DK100404.References
[1] M. Freiman et al., “Characterization of fast and slow diffusion from diffusion-weighted MRI of pediatric Crohn’s disease,” J. Magn. Reson. Imaging, vol. 37, no. 1, pp. 156–63, Jan. 2013.
[2] S. Kurugol et al., “Evaluation of Motion-Compensated Spatially-Constrained IVIM (MC-SCIM) Model of Diffusion-weighted MRI for Assessment of Fibrosis in Crohn’s Disease using Surgical Histopathology Scores,” in Proc. Intl. Soc. Mag. Reson. Med 2017, 2017.
[3] S. Kurugol, B. Marami, O. Afacan, S. K. Warfield, and A. Gholipour, “Motion-Robust Spatially Constrained Parameter Estimation in Renal Diffusion-Weighted MRI by 3D Motion Tracking and Correction of Sequential Slices,” in International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI), 2017, vol. 10555, pp. 75–85.
[4] M. Freiman, S. D. Voss, R. V Mulkern, J. M. Perez-Rossello, M. J. Callahan, and S. K. Warfield, “Reliable assessment of perfusivity and diffusivity from diffusion imaging of the body.,” Int. Conf. Med. Image Comput. Comput. Interv., vol. 15, no. Pt 1, pp. 1–9, 2012.
[5] D. Le Bihan, E. Breton, D. Lallemand, M. L. Aubin, J. Vignaud, and M. Laval-Jeantet, “Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging.,” Radiology, vol. 168, no. 2, pp. 497–505, Aug. 1988.
[6] D. Gallichan, J. L. R. Andersson, M. Jenkinson, M. D. Robson, and K. L. Miller, “Reducing distortions in diffusion-weighted echo planar imaging with a dual-echo blip-reversed sequence,” Magn. Reson. Med., vol. 64, no. 2, pp. 382–390, Aug. 2010.
[7] O. Afacan, W. S. Hoge, T. E. Wallace, A. Gholipour, S. Kurugol, and S. K. Warfield, “Dual-echo blip reversed EPI acquisition enables distortion correction in the presence of motion in diffusion-weighted MRI,” in International Society for Magnetic Resonance in Medicine (ISMRM), 2019, vol. 64, no. 2, pp. 3–5.
[8] J. L. R. Andersson, S. Skare, and J. Ashburner, “How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging,” Neuroimage, vol. 20, no. 2, pp. 870–888, Oct. 2003.
[9] S. Kurugol et al., “Motion Compensated Abdominal Diffusion Weighted MRI by Simultaneous Image Registration and Model Estimation (SIR-ME),” in International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI), vol. 9351, NIH Public Access, 2015, pp. 501–509.
[10] R. Davidson and E. Flachaire, “The wild bootstrap, tamed at last,” J. Econom., vol. 146, no. 1, pp. 162–169, Sep. 2008.
Figures