0970
Multidimensional correlation MRI of the brain
Kristofor Pas1,2, Michal Komlosh2,3, Daniel Perl4, Peter Basser2, and Dan Benjamini2,3
1University of Texas at Arlington, Arlington, TX, United States, 2National Institutes of Health, Bethesda, MD, United States, 3Uniformed Service University of the Health Sciences, Bethesda, MD, United States, 4Uniformed Services University of the Health Sciences, Bethesda, MD, United States
1University of Texas at Arlington, Arlington, TX, United States, 2National Institutes of Health, Bethesda, MD, United States, 3Uniformed Service University of the Health Sciences, Bethesda, MD, United States, 4Uniformed Services University of the Health Sciences, Bethesda, MD, United States
Synopsis
Multidimensional correlation MRI is an emerging imaging modality that is capable of disentangling highly heterogeneous systems, according to chemical and physical interactions of water within them. Using this approach, the conventional three dimensional MR scalar images are replaced with spatially resolved multidimensional spectra. The ensuing abundance in microstructural and chemical information is a blessing that incorporates a real challenge: how does one distill and refine it into images? Here we introduce a method that robustly identifies the multidimensional spectral components in the image domain, defines the spectral regions of interest, and uses them to reconstruct images of sub-voxel components.
Introduction
Most clinical MRI applications provide voxel-averaged scalar quantities that provide macroscopic information with respect to the voxel size, which is typically on the order of millimeters. One particular approach to overcome MRI's limited spatial resolution is to harness this technology's original designation as a spectroscopic modality. Achieving that goal, a series of advances within the field of porous media NMR have led to the conception of multidimensional correlation MR.1-7 This phenomenological approach makes no assumptions about tissue structure, and jointly encodes and processes different dynamic processes, such as relaxation and diffusion. The result is a joint multidimensional distribution of these dynamic properties and their correlations.Integration of this multidimensional approach within imaging applications was not feasible until recently due to burdensome data requirements resulting in impractically long scan times. The development of the marginal distributions constrained optimization (MADCO) framework8 minimized data requirements, leading to the feasibility of multidimensional correlation MR imaging. Although the spatial resolution of MRI is low compared with biological tissue local heterogeneity, multidimensional MRI provides a multicomponent signature in each voxel, which can report on physical microstructure and chemical composition of a range of tissue types, from animal spinal cord9-10 to human placenta.11
Multidimensional techniques are powerful, however, they also present significant challenges, and in particular, dimensionality reduction, which can be achieved by finding spectral regions of interest (S-ROIs) that contain peaks, and summing (i.e., numerically integrating) over them. Identifying the correct S-ROIs based on which multidimensional spectral images can be generated is probably the most pressing issue in this context, which we address here.
Theory
We present here an S-ROI generator that is designed to preserve inter-voxel variability and prevent spectral blurring that is caused by using the conventional approach of spatial averaging.10,11 Our guiding notion is that every spectral component may be valuable, regardless of its prevalence or its amplitude in the image and spectral domains, respectively. The proposed method gives equal weight to all spectral components across the image, thus preserving the information they represent. Without loss of generality, we choose to focus on 2D correlation distributions in this work. Fig. 1 presents an overview of the proposed approach, compared with the conventional method.Materials and Methods
A portion of occipital lobe was obtained from a brain specimen that was derived from the Uniformed Services University/Center for Neuroscience and Regenerative Medicine (USU/CNRM) Brain Tissue Repository collection (U.S. Department of Defense, Bethesda, Maryland, USA). This facility operates under the auspices of the USU Institutional Review Board, under an approved protocol. Following formalin fixation, the brain was serially sectioned in the coronal plane. A segment of occipital lobe containing V1 was then subdissected and used for this study.The cortical brain specimen was imaged in a 7 T Bruker vertical bore microimaging scanner using a 3D inversion recovery spin-echo diffusion-weighted (DW) echo planar imaging (IR-DWI-EPI) sequence and the following spatial parameters: field of view 33x26x18 mm3 with isotropic voxel dimension of 300 μm. This multidimensional acquisition encodes simultaneously T1-T2-<D>, according to the MADCO framework encoding scheme,8,9 using a total of 274 acquisitions to ensure orientationally averaged DW signal (where <D> is the orientationally averaged diffusivity).12
Results and Discussion
Conventional approach - spatial averaging. The conventional approach of identifying the S-ROIs was used by averaging the spectra across all image voxels, which resulted in <FT1-<D>>, <FT2-<D>>, and <FT1-T2> distributions (Fig. 2, top row). The identified S-ROIs in each case are marked and labeled, and integration over them resulted images of the corresponding spectral components (Fig. 2, bottom row). Looking at these images, some of them contained highly saturated regions, which indicate loss of spectral resolution. Specifically, only 2 distinct T2-<D> peaks were captured, contrary to a large body of research.3,5,13-15 Furthermore, the T2 dimension in both T2-<D> and T1-T2 is redundant, and does not provide any additional information.New approach - single voxel contributions. The same 3 sets of voxelwise 2D spectra were processed using the proposed automatic S-ROI generator algorithm, which yielded the images in Fig. 3. It is clear that the S-ROI generator preserved more spectral components, has identified a significantly larger number of multidimensional peaks, and the spatial maps did not contain over-saturated regions.
Based on previous studies,4,15,5,9,16-17 some of these images can be assigned to a physical or biological component with relatively high certainty. The long T1-fast <D> and the long T1-long T2 images are likely to be associated with unrestricted bulk water, while the short T1-short T2 and the short T1-slow <D> images are presumably associated with myelin. Additionally, clear separation of GM and WM is evident in some images, where, for example, the inter T2-slow <D> and long T1-inter <D> images exhibit hyperintensities primarily in WM, while the inter T1-fast <D> image is more specific to the cortical GM.
Conclusion
This work proposed and evaluated a robust framework for processing of multidimensional correlation MRI data that preserves spectral and image resolution while performing the required dimensionality reduction and image reconstruction. Using a robust processing framework allowed us to apply this new phenomenological imaging modality to generate multiple images of intra-voxel components in the human brain, thus potentially overcoming spatial resolution limitations.Acknowledgements
This work was supported by funds provided by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number ZIAHD000266) and the Center for Neuroregenerative Medicine (CNRM) under the auspices of the Henry Jackson Foundation (HJF). KP would like to thank Dr. Robert Lutz for his administration of the Biomedical Engineering Summer Internship Program (BESIP) and for providing the opportunity to work at NIH.References
- Kroeker, R. M. & Mark Henkelman, R. Analysis of biological NMR relaxation data with continuous distributions of relaxation times. J. Magn. Reson. (1969) 69, 218–235 (1986).
- English, A. E., Whittall, K. P., Joy, M. L. G. & Henkelman, R. M. Quantitative Two-Dimensional time Correlation Relaxometry. Magn. Reson. Medicine 22, 425–434 (1991).
- Beaulieu, C., Fenrich, F. R. & Allen, P. S. Multicomponent water proton transverse relaxation and T2-discriminated water diffusion in myelinated and nonmyelinated nerve. Magn. Reson. Imaging 16, 1201–10 (1998).
- Pfeuffer, J., Provencher, S. W. & Gruetter, R. Water diffusion in rat brain in vivo as detected at very large b values is multicompartmental. Magn. Reson. Mater. Physics, Biol. Medicine 8, 98–108 (1999).
- Does, M. D. & Gore, J. C. Compartmental study of T1 and T2 in rat brain and trigeminal nerve in vivo. Magn. Reson. Medicine 47, 274–283 (2002).
- Venkataramanan, L., Song, Y.-Q. & Hürlimann, M. D. Solving Fredholm integrals of the first kind with tensor product structure in 2 and 2.5 dimensions. IEEE Trans. Signal. Process. 50, 1017–1026 (2002).
- Dortch, R. D., Apker, G. A., Valentine, W. M., Lai, B. & Does, M. D. Compartment-specific enhancement of white matter and nerve ex vivo using chromium. Magn. Reson. Medicine 64, 688–97 (2010).
- Benjamini, D. & Basser, P. J. Use of marginal distributions constrained optimization (MADCO) for accelerated 2D MRI relaxometry and diffusometry. J. Magn. Reson. 271, 40–45 (2016).
- Benjamini, D. & Basser, P. J. Magnetic resonance microdynamic imaging reveals distinct tissue microenvironments. NeuroImage 163, 183–196 (2017).
- Kim, D., Doyle, E. K., Wisnowski, J. L., Kim, J. H. & Haldar, J. P. Diffusion-relaxation correlation spectroscopic imaging: A multidimensional approach for probing microstructure. Magn. Reson. Medicine 78, 2236–2249 (2017).
- Slator, P. J. et al. Combined diffusion-relaxometry MRI to identify dysfunction in the human placenta. Magn. Reson. Medicine 82, 95–106 (2019).
- Avram, A. V., Sarlls, J. E., Hutchinson, E. & Basser, P. J. Efficient experimental designs for isotropic generalized diffusion tensor MRI (IGDTI). Magn. Reson. Medicine 79, 180–194 (2018).
- Vasilescu, V., Katona, E., Simplâceanu, V. & Demco, D. Water compartments in the myelinated nerve. III. Pulsed NMR result. Experientia 34, 1443–1444 (1978).
- Whittall, K. & Mackay, A. Quantitative interpretation of nmr relaxation data. J. Magn. Reson. 84, 134–152 (1989).
- Peled, S., Cory, D. G., Raymond, S. A., Kirschner, D. A. & Jolesz, F. A. Water diffusion, T(2), and compartmentation in frog sciatic nerve. Magn. Reson. Medicine 42, 911–8 (1999).
- Ronen, I., Moeller, S., Ugurbil, K. & Kim, D.-S. Analysis of the distribution of diffusion coefficients in cat brain at 9.4 T using the inverse Laplace transformation. Magn. Reson. Imaging 24, 61–68 (2006).
- Benjamini, D. & Basser, P. J. Water mobility spectral imaging of the spinal cord: Parametrization of model-free Laplace MRI. Magn. Reson. Imaging 56, 187–193 (2019).
Figures
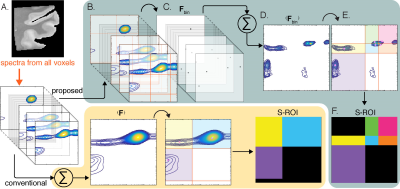
Figure 1. Overview of the proposed method, compared with the conventional approach. (A) Each voxel in a given image contains a 2D spectrum. Up to date, the strategy to process the voxelwise spectra into images was to average them,10,11 manually identify spectral components, and generate S-ROIs accordingly (bottom panel). We propose here to first (B) identify all possible spectral peaks in each voxel, and then (C) apply a threshold and obtain binary voxelwise spectra, Fbin. (D) These are then averaged to yield <Fbin>, (E) which is then used to generate the (F) S-ROIs.
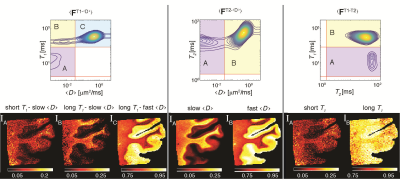
Figure 2. Conventional processing and image reconstruction of multidimensional correlation MRI data. T1-<D>, T2-<D>, and T1-T2 distributions averaged across the image were used to locate the S-ROIs and to generate the signal fraction images. Note the loss of spectral resolution that resulted in redundancy of the T2 dimension. Furthermore, many of the reconstructed images appear to be saturated, which implies the blending and smearing of the underlying spectral components.
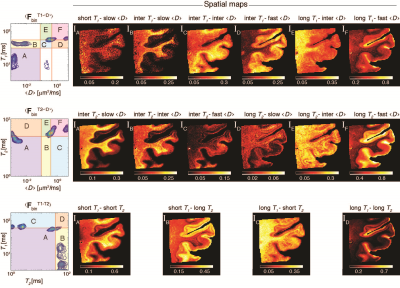
Figure 3. Proposed framework for processing and image reconstruction of multidimensional correlation MRI data. <FbinT1-<D>>, <FbinT2-<D>>, and <FbinT1-T2> were computed using the proposed algorithm in Fig. 1. These summarized spectra were then used to identify and define S-ROIs. Note that compared with the conventional approach, the S-ROI generator preserved more spectral components, has identified a significantly larger number of multidimensional peaks, and the spatial maps did not contain over-saturated region.