0964
Towards Single-shot Diffusion-weighted Spiral Abdominal Imaging on a Clinical Scanner1Philips Research Hamburg, Hamburg, Germany, 2Radiology, LUMC, Leiden, Netherlands, 3Philips Healtcare Best, Best, Netherlands
Synopsis
Single-shot diffusion-weighted imaging is predominantly performed with echo planar imaging today. Spiral imaging allows shorter echo times and thus promises higher SNR, but is more sensitive to various system imperfections. Previous work showed the feasibility of single-shot diffusion-weighted spiral imaging in the brain. This work explores the applicability to abdominal imaging. It shows that good image quality is achievable in volunteers, using the system demand trajectory for reconstruction, parallel imaging for acceleration, and static main field inhomogeneity mapping for corresponding deblurring.
Propose
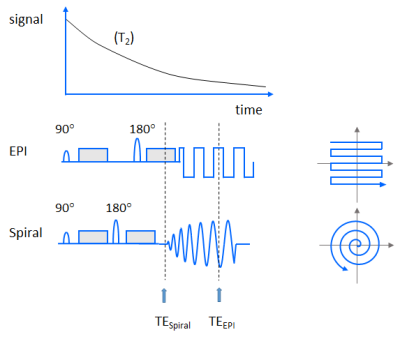
Diffusion has emerged as one of the most important contrasts in MRI1. By applying strong, pulsed magnetic field gradients, the micro-environment of the imaged water protons can be probed, serving important applications such as tumor diagnostics, stroke detection, and fiber tracking. However, these gradients render the acquisition sensitive to physiological motion, including respiration and heartbeat. Therefore, single-shot imaging is generally preferred1. Moreover, these gradients inevitably reduce the available signal, among others by prolonging the echo time (TE), which increases the transverse relaxation (T2)-induced signal decay. Spiral imaging allows shorter TEs and thus promises higher signal-to-noise ratio (SNR) (see Fig.1). However, spiral imaging is demanding in terms of gradient system performance and sensitive to blurring caused by main field inhomogeneities and T2*. The aim of the present work is to study the feasibility of single-shot diffusion-weighted (DW) abdominal spiral imaging on a clinical scanner without using extra field monitoring hardware2.Methods
Single-shot spin-echo diffusion-weighted spiral imaging with fat suppression was implemented on a 3T Ingenia Elition scanner (Philips Healthcare, Best, Netherlands) equipped with posterior and anterior body arrays (12+16 elements) and was evaluated in phantoms and 7 healthy volunteers. In this pilot study, typical spiral sequence parameters included a field of view of 420mm², an in-plane resolution of 3mm, a slice thickness of 5mm, a spiral acquisition window of 25 or 19ms, and a parallel imaging reduction factor of 3 or 4, respectively. For a b-value of up to 1000s/mm², a TE of 50ms was chosen. These basic settings were inspired by an existing, frequently used, clinical DW abdominal EPI protocol that uses the same voxel size, similar SENSE acceleration and sampling window (32ms) but allows only a shortest TE of 68ms (c.f. Fig.1). EPI was used for comparison to guide spiral image quality optimization. For spiral imaging, a numerically optimized gradient waveform was employed3, which provided uniform k-space undersampling in radial direction. Acquired data were processed with a regularized, iterative spiral SENSE reconstruction4, followed by field map-based deblurring5 with integrated Maxwell correction6. The field inhomogeneity (ΔB0) was estimated from a short, separate preparation scan collecting two in-phase gradient echoes. In these pilot volunteer experiments, fat-suppressed, B1+-shimmed acquisitions were performed with instructed breath-holds (in the end-expiratory state), acquiring three images with different b values (0, 100, 1000s/mm²), whereby magnitude signal averaging was performed after individual frame reconstruction for the highest b value.Results and Discussion
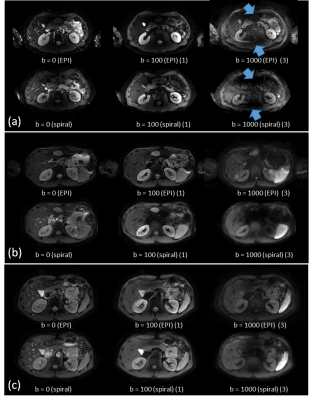
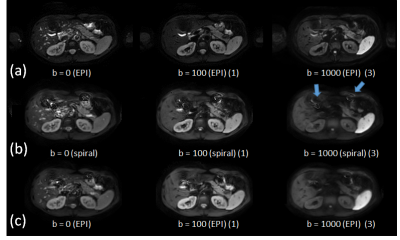
Phantom and volunteer experiments demonstrated the basic feasibility of abdominal single-shot diffusion-weighted spiral imaging on a clinical 3T system. Good image quality was achieved using the scanner’s k-space trajectory in gridding reconstruction for both non-DW and DW scans. Figure 2 shows selected, but representative results obtained in three of the volunteers. These indicate the need for B1+ shimming and a sufficiently high fidelity of the gradient system. Clearly visible is the increased liver signal in the spiral scans, due to the shorter TE. Further differences compared to EPI might result from different breath-hold positions, the different point-spread functions and their sensitivity to strong susceptibility. The overall quality of the spiral imaging results are comparable to those of the EPI results, although the spiral imaging results show a slight tendency of signal smoothing compared to the EPI results. This was addressed by shortening the spiral acquisition window using higher SENSE factors (c.f. Fig.3). Moreover, the accuracy and reproducibility of the f0 determination and the quality of the ΔB0 map proved to be essential in the abdominal region. This area is subject of respiratory motion in presence of a strong feet-head main field gradient caused by the strong susceptibility changes near the diaphragm. Thus, patient motion-induced field fluctuations represent the lower limit for the accuracy of the separate, static mapping but were sufficiently mitigated by the breath-holding regime.Conclusion
Single-shot abdominal diffusion-weighted spiral imaging is feasible on a clinical 3T platform. The performance and fidelity of the employed gradient system was found to be sufficient to dispense with extra hardware for field monitoring. Future work will have to further enhance image quality in this application. Moreover, the necessary field map acquisition needs to be integrated more seamlessly into a free breathing approach.Acknowledgements
No acknowledgement found.References
1. Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001; 13:534-546.
2. Wilm BJ, Barmet C, Gross S, et al. Single-shot spiral imaging enabled by an expanded encoding model: Demonstration in diffusion MRI. Magn Reson Med 2017; 77:83-91.
3. Pipe JG, Zwart NR. Spiral trajectory design: A flexible numerical algorithm and base analytical equations. Magn Reson Med 2014; 71:278–285.
4. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med 2001; 46:638-51.
5. Wang D, Zwart NR, Pipe JG. Joint water–fat separation and deblurring for spiral imaging. Magn Reson Med 2018; 79:3218–3228.
6. Börnert P, Schomberg H, Aldefeld B, Groen J. Improvements in spiral MR imaging. MAGMA 1999; 9:29-41.
Figures