0963
Acquisition of a reference Connectom diffusion MRI dataset: In vivo whole-brain diffusion MRI at 760 µm isotropic averaged over 18 hours1A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 33Department of Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States, 4Department of Radiology, Brigham and Women's Hospital, Boston, MA, United States, 5Department of Life Science Engineering, Institute of Medical Physics and Radiation Protection, Giessen, Germany
Synopsis
We present a whole brain in vivo diffusion MRI dataset acquired at 760um isotropic resolution and sampled at 1260 q-space points across 9 two-hour sessions. The creation of this benchmark in vivo diffusion MRI dataset is possible through use of the Connectom scanner, custom-built 64-channel phased-array coil, gSlider acquisition, dual-polarity GRAPPA reconstruction, reverse phase encoding for distortion mitigation, and personalized motion-robust stabilizer. The data will enhance our understanding of gray and white matter structure with fine detail revealed at sub-mm resolution and serve as a reference dataset for new modeling and processing algorithms.
Introduction
Diffusion MRI (dMRI) has proven to be an invaluable tool in the study of brain structural connectivity. However, dMRI has been limited to low spatial resolution due to technical challenges. With the advent of advanced acquisition/reconstruction, sub-millimeter isotropic resolution dMRI is becoming increasingly feasible, presenting new opportunities for studying brain structure at the mesoscopic scale. In this work, we aim to create a high-quality in-vivo dMRI reference dataset acquired at sub-millimeter resolution that will be made publicly available to the community, to aid in the exploration and evaluation of mesoscale structure revealed through in-vivo dMRI. This high-quality dataset may also be used as a test bed for new modeling, denoising and processing algorithms, potentially providing a common testing platform for further development.This reference dMRI dataset was acquired at 760um isotropic resolution using state-of-the-art hardware and acquisition techniques on the 3T Connectom scanner, with a carefully-designed diffusion protocol targeting a single-subject scan across 9 two-hour sessions. A custom-made form-based headcase (https://caseforge.co) was used to ensure consistency in subject positioning and B0 shim-settings across sessions for accurate registration between scans. White-matter and gray-matter diffusion results from our initial processing and analysis of this data nicely demonstrate its high SNR and high image quality.
Methods
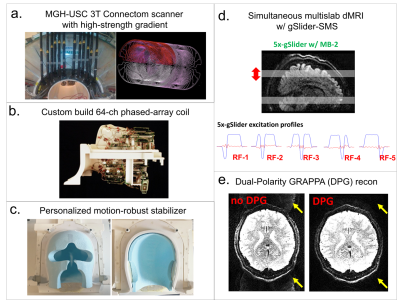
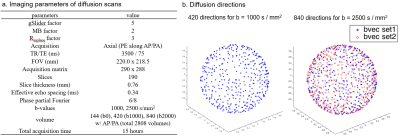
Acquisition: Data were acquired in a healthy volunteer on the MGH-USC 3T Connectom scanner with Gmax of 300 mT/m1-3 (Fig.1a), using a custom-built 64-channel phased-array coil4 (Fig.1b). The scan was completed in 9 two-hour scan sessions. A personalized motion-robust stabilizer (Fig.1c), that precisely fits the shape of the subject’s head, was used to ensure same subject positioning across sessions. B0 shim-settings were kept identical across sessions to minimize differences in image distortions, with the goal of minimizing reliance on image post-processing that could introduce blurring. gSlider-SMS5-6 was used as an SNR-efficient approach to acquire dMRI data (Fig.1d) with imaging parameters listed in Fig.2a. Two shells at b=1000s/mm2 and 2500s/mm2 were acquired as shown in Fig.2b, where the 420 directions at b=1000s/mm2 represented a subset of the 840 directions at b=2500s/mm2. A b=0s/mm2 image was acquired after every 10 diffusion images. For each volume, a paired reversed phase-encoding volume was also acquired. B0 map, B1 map, MPRAGE and T2-SPACE data were acquired on the subject.Reconstruction: Dual-Polarity-GRAPPA7-8 was used for parallel imaging reconstruction to minimize Nyquist ghost artifacts by correcting for higher-order phase errors (Fig.1e). POCS was used for partial Fourier reconstruction (p.f.=6/8) to recover high spatial information. Diffusion background phase-corruptions were removed using real-value diffusion algorithm9 to provide real-value data and avoid magnitude-bias in postprocessing steps. gSlider slab-to-slice reconstruction was performed with T1 correction using approach from10.
Postprocessing: Gradient non-linearity correction was performed using in-house Matlab code, following which susceptibility-induced and eddy current-induced distortion estimation and correction were performed using topup11 and the eddy-current correction toolbox12-14 in the FMRIB Software Library (FSL)15-17. DTI fitting and probabilistic diffusion model fitting18-19 was then performed, also using FSL. Finally, laminar cortical depth analysis was performed to evaluate radiality in grey matter, which is defined as the dot product of the primary diffusion direction at each vertex and the surface normal20-21.
Results
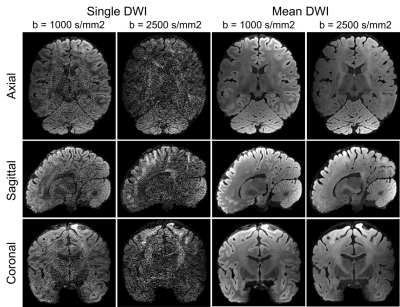
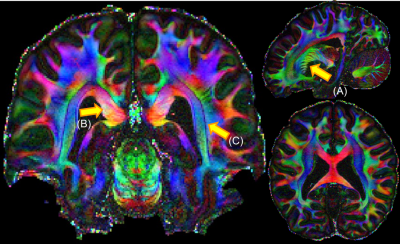
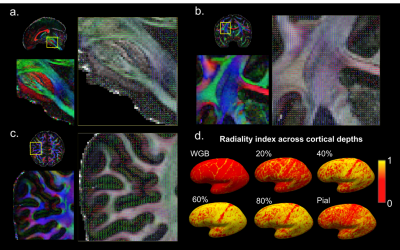
The single and mean DWI images in Fig.3 highlight the high SNR and robust image quality of this dataset in all spatial orientations. Color FA maps in Fig.4 reveal exquisite detail within fine-scaled structures, including (A) the gray matter bridges that span the internal capsule, giving rise to the characteristic stripes seen in the striatum; (B) the radiating fiber bundles within the corpus callosum, highlighted as red stripes on the FA map; and (C) the 1-voxel-width line of reduced-FA separating the corticospinal tract from an adjacent tract, highlighting the low level of blurring and high spatial accuracy of this data acquired across 9 two-hour sessions. In Fig.5, the complex fiber architecture in the pons, including the descending corticospinal tracts in the basis pontis (Fig.5a) and complex intermingling of fiber bundles within the centrum semiovale (Fig.5b), were detected in the high-resolution high-SNR data, demonstrating the ability to resolve crossing fibers, especially in resolving three-way crossings in the centrum semiovale formed by callosal fibers, corticospinal tract and superior longititudinal fasciculus. The depiction of cortical anisotropy (Fig.5c) consistently highlights the capability of visualizing the subcortical white matter as it turns into the cortex. Finally, the radiality calculated across different cortical depths provides reliable values that are higher in the middle-depth surfaces (40%-80% of the cortical thickness) but with a stripe of lower values in the somatosensory cortex (Fig.5d), showing the ability to capture sharp turning features across cortical depths. More extensive diffusion analysis results can be found at https://stuff.mit.edu/~fuyixue/, where the full dataset will be hosted after the postprocessing pipeline is finalized.Discussion and Conclusion
We present a high-quality sub-millimeter dMRI dataset made possible by leveraging state-of-art hardware and the latest advances in acquisition and reconstruction. The preliminary results presented here showcase the high SNR and high image quality, as well as the ability to visualize detailed brain structures and complex fiber architecture in vivo. Work is underway to further investigate the added value of such high-quality data to unveiling structural connectivity and microstructural complexity for understanding the structural basis of brain function.Acknowledgements
This work was supported by the NIH NIBIB (R01-EB020613, R01-EB019437, R01-MH116173, U01-EB025162, and U01EB026996) and the instrumentation Grants (S10-RR023401, S10-RR023043, and S10-RR019307).References
1. Setsompop K, Kimmlingen R, Eberlein E, Witzel T, Cohen-Adad J, McNab JA, Keil B, Tisdall MD, Hoecht P, Dietz P. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. Neuroimage 2013;80:220-233.
2. McNab JA, Edlow BL, Witzel T, Huang SY, Bhat H, Heberlein K, Feiweier T, Liu K, Keil B, Cohen-Adad J. The Human Connectome Project and beyond: initial applications of 300mT/m gradients. Neuroimage 2013;80:234-245.
3. Fan Q, Witzel T, Nummenmaa A, Van Dijk KR, Van Horn JD, Drews MK, Somerville LH, Sheridan MA, Santillana RM, Snyder J. MGH–USC Human Connectome Project datasets with ultra-high b-value diffusion MRI. Neuroimage 2016;124:1108-1114.
4. Keil B, Blau JN, Biber S, Hoecht P, Tountcheva V, Setsompop K, Triantafyllou C, Wald LL. A 64‐channel 3T array coil for accelerated brain MRI. Magnetic resonance in medicine 2013;70(1):248-258.
5. Setsompop K, Fan Q, Stockmann J, Bilgic B, Huang S, Cauley SF, Nummenmaa A, Wang F, Rathi Y, Witzel T. High‐resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider‐SMS). Magnetic Resonance in Medicine 2017.
6. Wang F, Bilgic B, Dong Z, Manhard MK, Ohringer N, Zhao B, Haskell M, Cauley SF, Fan Q, Witzel T. Motion‐robust sub‐millimeter isotropic diffusion imaging through motion corrected generalized slice dithered enhanced resolution (MC‐gSlider) acquisition. Magnetic resonance in medicine 2018;80(5):1891-1906.
7. Hoge WS, Polimeni JR. Dual‐polarity GRAPPA for simultaneous reconstruction and ghost correction of echo planar imaging data. Magnetic resonance in medicine 2016;76(1):32-44.
8. Hoge WS, Setsompop K, Polimeni JR. Dual‐polarity slice‐GRAPPA for concurrent ghost correction and slice separation in simultaneous multi‐slice EPI. Magnetic resonance in medicine 2018;80(4):1364-1375.
9. Eichner C, Cauley SF, Cohen-Adad J, Möller HE, Turner R, Setsompop K, Wald LL. Real diffusion-weighted MRI enabling true signal averaging and increased diffusion contrast. NeuroImage 2015;122:373-384.
10. Liao C, Stockmann J, Tian Q, Bilgic B, Arango NS, Manhard MK, Huang SY, Grissom WA, Wald LL, Setsompop K. High‐fidelity, high‐isotropic‐resolution diffusion imaging through gSlider acquisition with and T1 corrections and integrated ΔB0/Rx shim array. Magnetic resonance in medicine 2019;83(1):56-67.
11. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20(2):870-888.
12. Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016;125:1063-1078.
13. Andersson JL, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage 2016;141:556-572.
14. Andersson JL, Graham MS, Drobnjak I, Zhang H, Campbell J. Susceptibility-induced distortion that varies due to motion: correction in diffusion MR without acquiring additional data. Neuroimage 2018;171:277-295.
15. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208-S219.
16. Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage 2009;45(1):S173-S186.
17. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage 2012;62(2):782-790.
18. Jbabdi S, Sotiropoulos SN, Savio AM, Graña M, Behrens TE. Model‐based analysis of multishell diffusion MR data for tractography: How to get over fitting problems. Magnetic resonance in medicine 2012;68(6):1846-1855.
19. Hernández M, Guerrero GD, Cecilia JM, García JM, Inuggi A, Jbabdi S, Behrens TE, Sotiropoulos SN. Accelerating fibre orientation estimation from diffusion weighted magnetic resonance imaging using GPUs. PloS one 2013;8(4):e61892.
20. McNab JA, Polimeni JR, Wang R, Augustinack JC, Fujimoto K, Stevens A, Janssens T, Farivar R, Folkerth RD, Vanduffel W. Surface based analysis of diffusion orientation for identifying architectonic domains in the in vivo human cortex. Neuroimage 2013;69:87-100.
21. Fan Q, Nummenmaa A, Polimeni JR, Witzel T, Huang SY, Wedeen VJ, Rosen BR, Wald LL. HIgh b-value and high Resolution Integrated Diffusion (HIBRID) imaging. NeuroImage 2017;150:162-176.
Figures