0961
Overcoming geometric distortions in human prostate diffusion weighted imaging by spatio-temporal encoded (SPEN) MRI1Weizmann Institute of Science, Rehovot, Israel
Synopsis
Spatiotemporal encoding (SPEN) is an alternative ultrafast imaging technique which allows one to manipulate the bandwidth along the phase-encoding (PE) direction as well as to achieve T2* refocusing throughout the FID acquisition, thereby overcoming distortions observed along EPI’s PE dimension. The study compares multislice 2D SPEN and a 3D SPEN sequence variants against EPI derivatives, evaluating their ability to deliver prostate diffusion-weighted imaging (DWI) data and apparent diffusion coefficient (ADC) maps on healthy human volunteers. Essentially distortion-free diffusion weighted images and ADC maps of prostate with good SNR were achieved by the 2D SPEN variant.
INTRODUCTION
DWI and ADC mapping data have become an important part of the clinical toolkit currently used for the detection and characterization of prostate lesions by MRI.1 These are commonly retrieved using EPI-based sequences, for which immunity to distortions along PE direction is limited by the minimum available echo spacing. This can often lead to distortions for some of the slices collected in the prostate area. Parallel imaging and/or multi-segmented acquisitions can provide access to higher bandwidths (BWs) along the PE direction, this is often not sufficient to overcome distortions –particularly when imaging small objects, or scanning motion-prone abdominal regions. In the case of human prostate scans, this is further compounded by the presence of air/tissue/fat interfaces. This can lead to either image reconstruction artifacts and/or loss of resolution due to blurring. Spatiotemporal encoding2 (SPEN) is a single-shot technique that can overcome these shortcomings thanks to: (1) a PE BW that can be arbitrarily chosen at the time of the excitation, and (2) an ability to operate under fully T2*-refocusing conditions throughout the acquisition.3 Further, a recently proposed SPEN image reconstruction pipeline4 allows one to reconstruct multi-segmented diffusion weighted data collected with parallel receivers without suffering from motion-induced artifacts, opening an avenue to high resolution imaging of challenging anatomical regions. This study explores the use of these features for collecting single- and multi-shot SPEN-based 2D DW images and ADC maps of prostate. The study also explores the use of a 3D SPEN sequence, and compares its performance with respect to its 2D multislice counterpart for prostate imaging. Our results corroborate the aforementioned advantages of SPEN vs EPI and suggest that the former is a promising new alternative for prostate DWI.METHODS
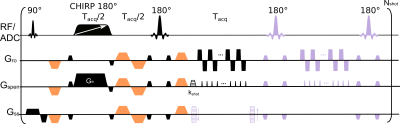
SPEN images were acquired using the multislice 2D and kz-encoded 3D sequences shown in Figure 1. Both 2D and 3D versions were tested for single shot and interleaved acquisitions along the PE direction. For the 3D sequence a navigator image for estimating potential phase corrections to be applied before FT along slab (z) dimension was acquired at the end of each scan, after rewinding any residual kz-encoding and after applying a 180° pulse to conserve SPEN’s T2* refocusing condition along the whole FOV. After correcting these potential phase distortions, a FT was performed along kz, followed by the SPEN processing scheme outlined in Ref. 4. The sequence in Figure 1 was programmed on a 3T Siemens Prisma scanner equipped with a 32 channels spine and 18 channels body coils. All images were acquired under free-breathing without respiratory gating. SPEN images were acquired with an encoding bandwidth of 8 kHz, slice thickness 3.5 mm, 16 slices or 16 kz encoding lines (2D/3D versions respectively), 3D FOV of 20x8x5.6 cm. For single-shot data 2x2 mm in-plane resolution was targeted with #PE=40, while for two-shot (interleaved) SPEN data a resolution of 1.5x1.5 mm was achieved (#PE=56). A partial Fourier factor of 0.8 was used along read-out (RO) dimension for the SPEN acquisitions. Single-shot EPI was acquired with 2x2 mm in-plane resolution using a PE BW of 1.8 kHz. Scanner-supplied RESOLVE5 with a 1.5x1.5 mm in-plane resolution using 5 segments along the RO dimension and a GRAPPA acceleration of 2 was also collected, resulting in an effective BW of 6 kHz along the PE dimension. For both EPI and RESOLVE the FOV along the PE direction was set to 20 cm to avoid folding; SPEN acquisitions are immune to folding and thus used a reduced FOV(PE) of 8 cm. For all of the DW experiments b-values of 50 and 800 s/mm2 were used with 3 and 9 repetitions, respectively for single-shot data, and 3 and 4 repetitions were used for the two-shot data. To keep the scan times comparable, 2 RESOLVE repetitions were acquired for each b-value to compensate for time necessary to accommodate 5 RO segments. All human volunteers were scanned following suitable written consent.RESULTS & DISCUSSIONS
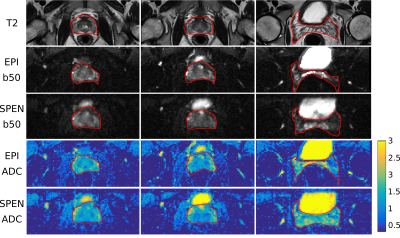
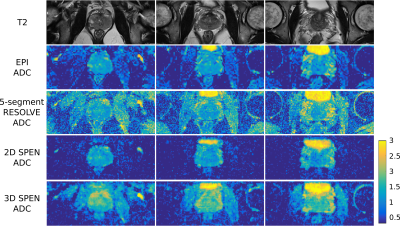
Figure 2 compares DWI and ADC maps acquired with the SPEN and EPI-based sequences, along with TSE-T2 weighted images to access true anatomy. Reduction of distortions can be appreciated in SPEN’s DWI and ADC maps, recovering signal for regions which suffer from pile-up artifacts in the EPI data. Figure 3 presents SPEN multishot acquisitions both for 2D multislice and 3D implementations, which once again corroborate SPEN’s potential to acquire high resolution ADC maps for prostate while being resilient to field inhomogeneity and/or motion induced distortions.CONCLUSION
2D multislice and 3D volumetric SPEN sequences were introduced, and their potential was evaluated within a prostate DWI context. Both SPEN sequences managed to overcome distortions observed in EPI-based images thanks to their higher BW and full refocusing, yielding results comparable to those arising from RESOLVE acquisitions that utilize a higher number of segments than their SPEN counterparts. These results are leading to studies on a larger cohort of healthy and pre-biopsy volunteers.Acknowledgements
We are grateful to Fanny Attar, Eiska Tegareh and Dr. Edna Furman-Haran for assistance in the scans. Financial support from the Kimmel Institute for Magnetic Resonance and the Thompson Family Foundation is gratefully acknowledged.References
1. Weinreba, J. C., Barentszb, J. O, Choyke, P. L. et al. (2016) PI-RADS Prostate Imaging – Reporting and Data System: 2015, Version 2. European Urology, 69,16–40.
2. Tal, A., Frydman, L. (2006) Spatial encoding and the single-scan acquisition of high definition MR images in inhomogeneous fields. Journal of Magnetic Resonance, 182, 179–194.
3. Schmidt, R., Frydman, L. (2014) New spatiotemporal approaches for fully refocused, multislice ultrafast 2D MRI. Magnetic Resonance in Medicine. 71, 711–722.
4. Cousin, S. F., Liberman, G., Solomon, E., Otikovs, M., Frydman, L. (2019) A regularized reconstruction pipeline for high‐definition diffusion MRI in challenging regions incorporating a per‐shot image correction. Magnetic Resonance in Medicine, 82, 1322–1330.
5. Porter, D. A., Heidemann, R. M. (2009) High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magnetic Resonance in Medicine, 62, 468–475.
6. Schmidt, R., Seginer, A., Frydman, L. (2016) Interleaved multishot imaging by spatiotemporal encoding: A fast, self-referenced method for high-definition diffusion and functional MRI: Referenceless Interleaved SPEN MRI. Magnetic Resonance in Medicine, 75, 1935–1948.
7. Liberman, G., Solomon, E., Lustig, M., Frydman, L. (2018) Multiple‐coil k‐space interpolation enhances resolution in single‐shot spatiotemporal MRI. Magnetic Resonance in Medicine, 79, 796–805.
Figures