0957
Motion-compensated gradient waveform design for tensor-valued diffusion encoding by constrained numerical optimization1Clinical Sciences Lund, Lund University, Lund, Sweden, 2Radiology, Brigham and Women's Hospital, Boston, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, United Kingdom
Synopsis
Motion compensation is vital for cardiac diffusion MRI. In this paper we propose an optimized gradient waveform design that allows tensor-valued diffusion encoding with motion compensation. We demonstrate that it works for in vivo cardiac imaging and we show that it is more efficient than previous designs.
Introduction
Tissue movement during diffusion encoding can lead to phase dispersion that is erroneously interpreted as an elevated diffusion coefficient. Although this movement is present in the brain, primarily due to arterial pulsation, it is most relevant in other organs where both cardiac and respiratory motion is prominent. To combat this artifact, diffusion encoding gradient waveforms have been designed to compensate for motion [1,2]. Recently, such waveforms for tensor-valued diffusion encoding [3] were suggested by Lasič et al. [4] based on a symmetric design.$$$~~~~~$$$In this work we propose a design that uses asymmetric numerically optimized gradient waveforms with nulling of arbitrary moments, and we show that this design improves the encoding efficiency and removes several technical limitations of previous designs.
Methods
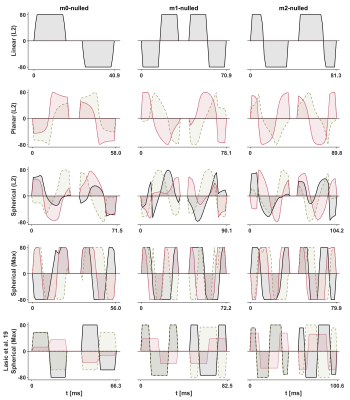
We generate gradient waveforms ($$$\mathbf{g}(t)$$$) by extending the optimization framework by Sjölund et al. [5] to include constraints on the waveform moments; in addition to the constraints on b-tensor shape, gradient amplitude, slew rate, energy consumption, and Maxwell compensation [6]. Moment nulling is achieved by minimizing the magnitude of the moment vector $$$\mathbf{m}$$$ of order $$$n$$$$$\mathbf{m}_n=\gamma \int_0^{\mathrm{TE}}\mathbf{g}(t)\cdot t^n\mathrm{d}t,$$
to a limit $$$||\mathbf{m}_n||<L_n$$$, where $$$\gamma$$$ is the gyromagnetic ratio and $$$t$$$ is the time. We note that n=1, n=2, n=3 correspond to velocity, acceleration and jerk, respectively. Unlike previous designs [4], the proposed waveforms (i) are not "self-balanced" ($$$||\mathbf{m}_0||\gg0$$$ after refocusing) which removes the need/influence of additional crusher gradients that disturb the motion compensation; (ii) use so called 'M-nulling' so that they can be arbitrarily rotated and distorted by gradient non-linearity without compromising the compensation for concomitant gradient effects [6,7].
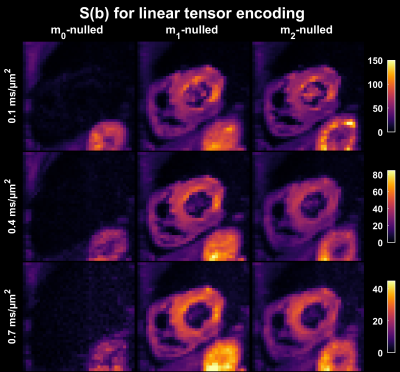
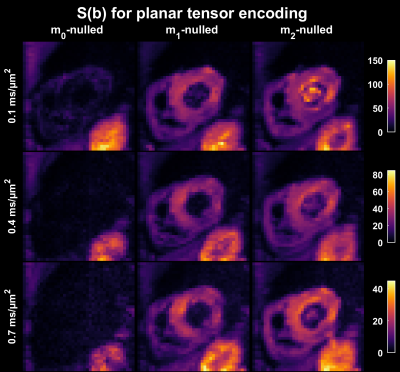
$$$~~~~~$$$To demonstrate the feasibility of this approach, motion compensated waveforms $$$(\mathbf{m}_0,~\mathbf{m}_1,~\mathbf{m}_2)$$$ that yield linear and planar b-tensors [3] were used for diffusion weighted imaging of a healthy heart in vivo. The moment limits were $$$L_0=0,~L_1=10^{-4},~L_2=10^{-4}$$$ in units of sn/m. For comparison, the magnitude of $$$\mathbf{m}_1$$$ and $$$\mathbf{m}_2$$$ for non-compensated waveforms are approximately eight orders of magnitude larger. MRI data were acquired in the heart of a healthy volunteer on a 3 T Prisma (Siemens Healthcare, Erlangen, Germany) using a prototype spin-echo EPI sequence with ZOOM-it excitation, TR = 5 s RR-intervals, TE = [77, 93, 99] ms, partial-Fourier = 6/8, in-plane resolution = 3×3 mm2, slice thickness = 8 mm, FOV = 320×118 mm2, b = [0.1, 0.4, 0.7] ms/µm2 in 6, 10, 15 directions (one repetition). The acquisition was cardiac triggered under free-breathing. The encoding duration was approximately 3 ms longer before than after the refocusing pulse.
$$$~~~~~$$$The impact of motion compensation in the heart was evaluated in terms of the diffusion weighted signal averaged over multiple rotations of planar and linear b-tensor encoding, as well as in terms of the calculated mean diffusivity (MD).
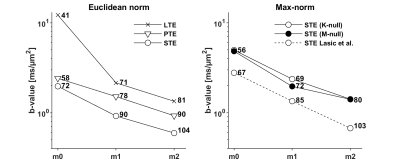
$$$~~~~~$$$The encoding efficiency of waveforms with variable limits on motion encoding was investigated in terms of the maximal b-value achievable for a fix encoding duration, and the necessary encoding time to reach b = 2 ms/µm2.
Results
The optimization framework could robustly generate motion compensated gradient waveforms for arbitrary shapes of the b-tensor (Fig. 1) and these could be executed in a cardiac imaging setting. Linear (Fig. 2) and planar b-tensor encoding (Fig. 3) showed a marked improvement from motion compensation; non-compensated waveforms exhibited a massive loss of signal whereas $$$\mathbf{m}_2$$$-nulled waveforms did not. The impact of this on quantification can be appreciated in the MD maps calculated from each set of data (Fig. 4).$$$~~~~~$$$As expected, the encoding performance was reduced as higher moment nulling was enforced (Fig. 5). However, the novel design outperformed the previous design, shortening the encoding times by up to 23 ms under the given premise. For a larger timing asymmetry, e.g. when ZOOM-it is not used, the advantage becomes even more pronounced (data not shown).
Discussion and conclusions
We have proposed and demonstrated a novel design for motion compensated gradient waveforms for tensor-valued diffusion encoding with significantly improved performance compared to previous designs. The suggested design has several benefits:- improved performance over previous designs (shorter echo-times and/or higher SNR)
- more flexible, allowing for arbitrary distribution of the encoding time
- automatically account for crushing and therefore achieve more accurate compensation
- ensures Maxwell compensation for arbitrary rotations of the waveforms
- ensures Maxwell compensation even for systems with prominent gradient non-linearity [6]
Acknowledgements
This work was supported by grants from Swedish Research Council (2016-03443). This work was supported by the British Heart Foundation, UK (SI/14/1/30718). We thank David Shelley for performing the MRI scan. We thank Siemens Healthcare (Erlangen, Germany) for access to the pulse sequence programming environment.References
[1] U. Gamper, P. Boesiger, and S. Kozerke, “Diffusion imaging of the in vivo heart using spin echoes-considerations on bulk motion sensitivity.” MRM, 2007.
[2] C. Stoeck, C. von Deuster, M. Genet, D. Atkinson, and S. Kozerke, “Second-order motion-compensated spin echo diffusion tensor imaging of the human heart.” MRM, 2016.
[3] C-F. Westin, H. Knutsson, O. Pasternak, F. Szczepankiewicz, E. Özarslan, D. van Westen, C. Mattisson, M. Bogren, L. O’Donnell, M. Kubicki, D. Topgaard, and M. Nilsson, “Q-space trajectory imaging for multidimensional diffusion MRI of the human brain.” MRM, 2019.
[4] S. Lasič, F. Szczepankiewicz, E. Dall’Armellina, A. Das, C. Kelly, S. Plein, J. E. Schneider, M. Nilsson, and I. Teh, “Motion compensated b-tensorencoding for in vivo cardiac diffusion-weighted imaging.” NMR in Biomed, 2019.
[5] J. Sjölund, F. Szczepankiewicz, M. Nilsson, D. Topgaard, C.-F. Westin, and H. Knutsson, “Constrained optimization of gradient waveforms for generalized diffusion encoding,” JMR, 2015.
[6] F. Szczepankiewicz, C.-F. Westin, and M. Nilsson, “Maxwell-compensated design of asymmetric gradient waveforms for tensor-valued diffusion encod-ing.” MRM, 2019.
[7] F. Szczepankiewicz, C. Eichner, A. Anwander, C.-F. Westin, and M. Paquette, “The effect of concomitant fields caused by gradient non-linearity when using M- and K-nulled maxwell compensation,” Proc. Int. Soc. Magn. Reson. Med. (no. 1547, submitted), 2020.
[8] F. Nery, F. Szczepankiewicz, L. Kerkelä, M. G. Hall, E. Kaden, I. Gordon,D. L. Thomas, and C. A. Clark, “In vivo demonstration of microscopic anisotropy in the human kidney using multidimensional diffusion MRI." MRM, 2019.
Figures